Enhanced CD36 scavenger receptor expression in THP-1 human monocytes in the presence of lupus plasma: linking autoimmunity and atherosclerosis
- PMID: 19144874
- PMCID: PMC4362773
- DOI: 10.3181/0806-BC-194
Enhanced CD36 scavenger receptor expression in THP-1 human monocytes in the presence of lupus plasma: linking autoimmunity and atherosclerosis
Abstract
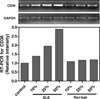
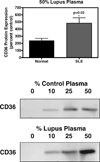
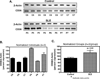
Premature atherosclerotic cardiovascular disease (ASCVD) is a common and devastating complication of systemic lupus erythematosus (SLE). It is likely that immunologic derangements contribute to premature ASCVD in these patients, possibly by disrupting homeostatic mechanisms that orchestrate cholesterol balance in monocytes/macrophages in the artery wall. CD36, a macrophage scavenger receptor responsible for recognition and internalization of oxidized lipids, is a major participant in atherosclerotic foam cell formation. We hypothesized that lupus plasma would affect CD36 expression in a pro-atherogenic manner in THP-1 human monocytes and differentiated macrophages. SLE patient plasma markedly stimulated expression of CD36 message in a dose-dependent fashion in THP-1 human monocytes. A 50% volume/volume concentration of plasma derived from SLE patients increased CD36 mRNA by 71 +/- 8% (n = 3, P < 0.001) above 50% normal human plasma. 50% SLE patient plasma increased CD36 mRNA expression to 290 +/- 12% of no-plasma control (n = 3, P < 0.001), compared with only 118 +/- 3.7% of control in the presence of 50% normal human plasma (n = 3, not significant). 50% lupus plasma also upregulated CD36 protein expression by 482.3 +/- 76.2% (n = 4, P < 0.05), whereas the presence of 50% normal human plasma increased the CD36 protein level by only 239.8 +/- 61.9% (n = 4, P < 0.05). To our knowledge, this is the first demonstration that CD36 expression is enhanced by plasma from patients with an autoimmune disorder. Premature atherosclerosis is common in SLE patients. Upregulation of CD36 may contribute to this pathological process by increasing vulnerability to cholesterol overload. Demonstration of disrupted cholesterol homeostasis in this select group of patients provides further evidence of the involvement of the immune system in atherogenesis and may inform us of the role of CD36 in the general atherogenic process. CD36 may provide a novel therapeutic target in the treatment of ASCVD in SLE patients.
Figures


References
-
- Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, Linton MF, Raggi P, Stein CM. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. - PubMed
-
- Hahn B, McMahon M. Atherosclerosis and systemic lupus erythematosus: the role of altered lipids and of autoantibodies. Lupus. 2008;17:368–370. - PubMed
-
- Frostegard J. Systemic lupus erythematosus and cardiovascular disease. Lupus. 2008;17:364–367. - PubMed
-
- Esdaile JM, Panaritis C, Abrahamowicz M. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. - PubMed

