TRPA1 acts as a cold sensor in vitro and in vivo
- PMID: 19144922
- PMCID: PMC2633575
- DOI: 10.1073/pnas.0808487106
TRPA1 acts as a cold sensor in vitro and in vivo
Abstract
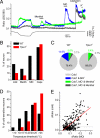
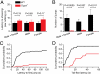
TRPA1 functions as an excitatory ionotropic receptor in sensory neurons. It was originally described as a noxious cold-activated channel, but its cold sensitivity has been disputed in later studies, and the contribution of TRPA1 to thermosensing is currently a matter of strong debate. Here, we provide several lines of evidence to establish that TRPA1 acts as a cold sensor in vitro and in vivo. First, we demonstrate that heterologously expressed TRPA1 is activated by cold in a Ca(2+)-independent and Ca(2+) store-independent manner; temperature-dependent gating of TRPA1 is mechanistically analogous to that of other temperature-sensitive TRP channels, and it is preserved after treatment with the TRPA1 agonist mustard oil. Second, we identify and characterize a specific subset of cold-sensitive trigeminal ganglion neurons that is absent in TRPA1-deficient mice. Finally, cold plate and tail-flick experiments reveal TRPA1-dependent, cold-induced nociceptive behavior in mice. We conclude that TRPA1 acts as a major sensor for noxious cold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

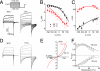


References
-
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. - PubMed
-
- Talavera K, Nilius B, Voets T. Neuronal TRP channels: Thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31:287–295. - PubMed
-
- Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. - PubMed
-
- Colburn RW, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. - PubMed
-
- Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

