Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm
- PMID: 19144954
- PMCID: PMC2804839
- DOI: 10.1095/biolreprod.108.075242
Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm
Abstract
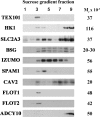
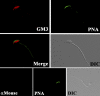
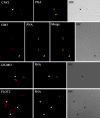
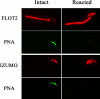
Mammalian sperm become fertile after completing capacitation, a process associated with cholesterol loss and changes in the biophysical properties of the sperm membranes that prepares the sperm to undergo the acrosome reaction. Different laboratories have hypothesized that cholesterol efflux can influence the extent and/or movement of lipid raft microdomains. In a previous study, our laboratory investigated the identity of sperm proteins putatively associated with rafts. After extraction with Triton X-100 and ultracentrifugation in sucrose gradients, proteins distributing to the light buoyant-density fractions were cored from polyacrylamide gels and microsequenced. In this study, a subset of these proteins (TEX101, basigin, hexokinase 1, facilitated glucose transporter 3, IZUMO, and SPAM1) and other molecules known to be enriched in membrane rafts (caveolin 2, flotillin 1, flotillin 2, and the ganglioside GM3) were selected to investigate their localization in the sperm and their behavior during capacitation and the acrosome reaction. These molecules localize to multiple sperm domains, including the acrosomal cap (IZUMO, caveolin 2, and flotillin 2), equatorial segment (GM3), cytoplasmic droplet (TEX101), midpiece (basigin, facilitated glucose transporter 3, and flotillin 2), and principal piece (facilitated glucose transporter 3). Some of these markers modified their immunofluorescence pattern after sperm incubation under capacitating conditions, and these changes correlated with the occurrence of the acrosome reaction. While GM3 and caveolin 2 were not detected after the acrosome reaction, flotillin 2 was found in the equatorial segment of acrosome-reacted sperm, and IZUMO distributed along the sperm head, reaching the post- and para-acrosomal areas. Taking into consideration the requirement of the acrosome reaction for sperm to become fusogenic, these results suggest that membrane raft dynamics may have a role in sperm-egg membrane interaction.
Figures
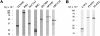


References
-
- Yanagimachi R.Mammalian fertilization. Knobil E, Neill JD.The Physiology of Reproduction, vol. 1, 2nd ed New York:Raven Press;1994: 189–317.
-
- Visconti PE, Kopf GS.Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59: 1–6. - PubMed
-
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS.Capacitation of mouse spermatozoa, I: correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129–1137. - PubMed
-
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS.Capacitation of mouse spermatozoa, II: protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995; 121: 1139–1150. - PubMed
-
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS.Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999; 214: 429–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

