Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo
- PMID: 19144994
- PMCID: PMC2666176
- DOI: 10.1194/jlr.M800622-JLR200
Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo
Abstract
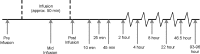
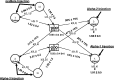
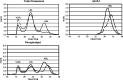
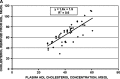
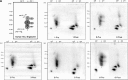
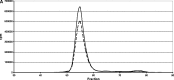
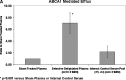
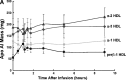
Uptake of cholesterol from peripheral cells by nascent small HDL circulating in plasma is necessary to prevent atherosclerosis. This process, termed reverse cholesterol transport, produces larger cholesterol-rich HDL that transfers its cholesterol to the liver facilitating excretion. Most HDL in plasma is cholesterol-rich. We demonstrate that treating plasma with a novel selective delipidation procedure converts large to small HDL [HDL-selectively delipidated (HDL-sdl)]. HDL-sdl contains several cholesterol-depleted species resembling small alpha, prebeta-1, and other prebeta forms. Selective delipidation markedly increases efficacy of plasma to stimulate ABCA1-mediated cholesterol transfer from monocytic cells to HDL. Plasma from African Green monkeys underwent selective HDL delipidation. The delipidated plasma was reinfused into five monkeys. Prebeta-1-like HDL had a plasma residence time of 8 +/- 6 h and was converted entirely to large alpha-HDL having residence times of 13-14 h. Small alpha-HDL was converted entirely to large alpha-HDL. These findings suggest that selective HDL delipidation activates reverse cholesterol transport, in vivo and in vitro. Treatment with delipidated plasma tended to reduce diet-induced aortic atherosclerosis in monkeys measured by intravascular ultrasound. These findings link the conversion of small to large HDL, in vivo, to improvement in atherosclerosis.
Figures






References
-
- Barr D. P., E. M. Russ, and H. A. Eder. 1951. Protein-lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am. J. Med. 11 480–493. - PubMed
-
- Gofman J. W., W. Young, and R. Tandy. 1966. Ischemic heart disease, Atherosclerosis, and longevity. Circulation. 34 679–697. - PubMed
-
- Miller G. J., and N. E. Miller. 1975. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1 16–19. - PubMed
-
- Gordon D. J., J. L. Probstfield, R. J. Garrison, J. D. Neaton, W. P. Castelli, J. D. Knoke, D. R. Jacobs, S. Bangdiwala, and H. A. Tyroler. 1989. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 79 8–15. - PubMed
-
- Sanossian N., J. L. Saver, M. Navab, and B. Ovbiagele. 2007. High density lipoprotein cholesterol. An emerging target for stroke treatment. Stroke. 38 1104–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

