Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses
- PMID: 19145246
- PMCID: PMC2615220
- DOI: 10.1371/journal.pone.0004204
Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses
Abstract
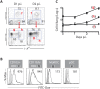
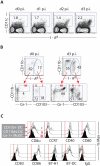
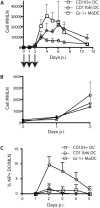
Dendritic cells located at the body surfaces, e.g. skin, respiratory and gastrointestinal tract, play an essential role in the induction of adaptive immune responses to pathogens and inert antigens present at these surfaces. In the respiratory tract, multiple subsets of dendritic cells (RDC) have been identified in both the normal and inflamed lungs. While the importance of RDC in antigen transport from the inflamed or infected respiratory tract to the lymph nodes draining this site is well recognized, the contribution of individual RDC subsets to this process and the precise role of migrant RDC within the lymph nodes in antigen presentation to T cells is not clear. In this report, we demonstrate that two distinct subsets of migrant RDC--exhibiting the CD103(+) and CD11b(hi) phenotype, respectively--are the primary DC presenting antigen to naïve CD4(+) and CD8(+) T lymphocytes in the draining nodes in response to respiratory influenza virus infection. Furthermore, the migrant CD103(+) RDC subset preferentially drives efficient proliferation and differentiation of naive CD8(+) T cells responding to infection into effector cells, and only the CD103(+) RDC subset can present to naïve CD8(+) T cells non-infectious viral vaccine introduced into the respiratory tract. These results identify CD103(+) and CD11b(hi) RDC as critical regulators of the adaptive immune response to respiratory tract infection and potential targets in the design of mucosal vaccines.
Conflict of interest statement
Figures



References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Henri S, Vremec D, Kamath A, Waithman J, Williams S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. - PubMed
-
- Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin Immunol. 2005;17:262–272. - PubMed
-
- Stumbles PA, Upham JW, Holt PG. Airway dendritic cells: co-ordinators of immunological homeostasis and immunity in the respiratory tract. Apmis. 2003;111:741–755. - PubMed
-
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

