Abeta42-induced neurodegeneration via an age-dependent autophagic-lysosomal injury in Drosophila
- PMID: 19145255
- PMCID: PMC2626277
- DOI: 10.1371/journal.pone.0004201
Abeta42-induced neurodegeneration via an age-dependent autophagic-lysosomal injury in Drosophila
Abstract
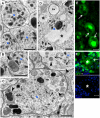
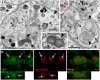
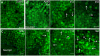
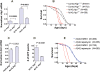
The mechanism of widespread neuronal death occurring in Alzheimer's disease (AD) remains enigmatic even after extensive investigation during the last two decades. Amyloid beta 42 peptide (Abeta(1-42)) is believed to play a causative role in the development of AD. Here we expressed human Abeta(1-42) and amyloid beta 40 (Abeta(1-40)) in Drosophila neurons. Abeta(1-42) but not Abeta(1-40) causes an extensive accumulation of autophagic vesicles that become increasingly dysfunctional with age. Abeta(1-42)-induced impairment of the degradative function, as well as the structural integrity, of post-lysosomal autophagic vesicles triggers a neurodegenerative cascade that can be enhanced by autophagy activation or partially rescued by autophagy inhibition. Compromise and leakage from post-lysosomal vesicles result in cytosolic acidification, additional damage to membranes and organelles, and erosive destruction of cytoplasm leading to eventual neuron death. Neuronal autophagy initially appears to play a pro-survival role that changes in an age-dependent way to a pro-death role in the context of Abeta(1-42) expression. Our in vivo observations provide a mechanistic understanding for the differential neurotoxicity of Abeta(1-42) and Abeta(1-40), and reveal an Abeta(1-42)-induced death execution pathway mediated by an age-dependent autophagic-lysosomal injury.
Conflict of interest statement
Figures
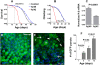
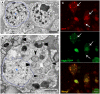
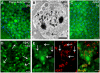
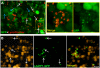
Similar articles
-
Brain aging and Aβ₁₋₄₂ neurotoxicity converge via deterioration in autophagy-lysosomal system: a conditional Drosophila model linking Alzheimer's neurodegeneration with aging.Acta Neuropathol. 2011 Feb;121(2):183-91. doi: 10.1007/s00401-010-0772-0. Epub 2010 Nov 14. Acta Neuropathol. 2011. PMID: 21076961
-
Accumulation of amyloid-like Aβ1-42 in AEL (autophagy-endosomal-lysosomal) vesicles: potential implications for plaque biogenesis.ASN Neuro. 2014 Mar 12;6(2):e00139. doi: 10.1042/AN20130044. ASN Neuro. 2014. PMID: 24521233 Free PMC article.
-
LC3 overexpression reduces Aβ neurotoxicity through increasing α7nAchR expression and autophagic activity in neurons and mice.Neuropharmacology. 2015 Jun;93:243-51. doi: 10.1016/j.neuropharm.2015.02.003. Epub 2015 Feb 14. Neuropharmacology. 2015. PMID: 25686800
-
Autophagic dysfunction in Alzheimer's disease: Cellular and molecular mechanistic approaches to halt Alzheimer's pathogenesis.J Cell Physiol. 2019 Jun;234(6):8094-8112. doi: 10.1002/jcp.27588. Epub 2018 Oct 26. J Cell Physiol. 2019. PMID: 30362531 Review.
-
Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review.Free Radic Res. 2002 Dec;36(12):1307-13. doi: 10.1080/1071576021000049890. Free Radic Res. 2002. PMID: 12607822 Review.
Cited by
-
D159 and S167 are protective residues in the prion protein from dog and horse, two prion-resistant animals.Neurobiol Dis. 2018 Nov;119:1-12. doi: 10.1016/j.nbd.2018.07.011. Epub 2018 Jul 24. Neurobiol Dis. 2018. PMID: 30010001 Free PMC article.
-
The Regulation of microRNAs in Alzheimer's Disease.Front Neurol. 2020 Apr 17;11:288. doi: 10.3389/fneur.2020.00288. eCollection 2020. Front Neurol. 2020. PMID: 32362867 Free PMC article. Review.
-
Autophagy and neuronal cell death in neurological disorders.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a008839. doi: 10.1101/cshperspect.a008839. Cold Spring Harb Perspect Biol. 2012. PMID: 22983160 Free PMC article. Review.
-
Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43.Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):15024-9. doi: 10.1073/pnas.1206362109. Epub 2012 Aug 29. Proc Natl Acad Sci U S A. 2012. PMID: 22932872 Free PMC article.
-
Alzheimer's disease: diverse aspects of mitochondrial malfunctioning.Int J Clin Exp Pathol. 2010 Jun 25;3(6):570-81. Int J Clin Exp Pathol. 2010. PMID: 20661404 Free PMC article. Review.
References
-
- Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. - PubMed
-
- Laferla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. - PubMed
-
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol Aging. 2005;26:1235–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

