Transition-state geometry measurements from (13)c isotope effects. The experimental transition state for the epoxidation of alkenes with oxaziridines
- PMID: 19146405
- PMCID: PMC2640442
- DOI: 10.1021/ja8088636
Transition-state geometry measurements from (13)c isotope effects. The experimental transition state for the epoxidation of alkenes with oxaziridines
Abstract
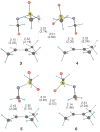
We here suggest and evaluate a methodology for the measurement of specific interatomic distances from a combination of theoretical calculations and experimentally measured (13)C kinetic isotope effects. This process takes advantage of a broad diversity of transition structures available for the epoxidation of 2-methyl-2-butene with oxaziridines. From the isotope effects calculated for these transition structures, a theory-independent relationship between the C-O bond distances of the newly forming bonds and the isotope effects is established. Within the precision of the measurement, this relationship in combination with the experimental isotope effects provides a highly accurate picture of the C-O bonds forming at the transition state. The diversity of transition structures also allows an evaluation of the Schramm process for defining transition-state geometries on the basis of calculations at nonstationary points, and the methodology is found to be reasonably accurate.
Figures

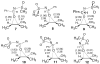
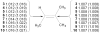

References
-
-
For an exception, see: Wenthold PG, Hrovat DA, Borden WT, Lineberger WC. Science. 1996;272:1456–1459.
-
-
- Bigeleisen J, Wolfsberg M. Advances in chemical physics. Vol. 1. Interscience; New York: 1958. pp. 16–76.
- Melander L, Saunders WH., Jr . Isotope effects on reaction rates. Wiley-Interscience; New York: 1980.
- More O’ Ferrall RA. J Chem Soc B. 1970:785–790.
- Katz AM, Saunders WH., Jr J Am Chem Soc. 1969;91:4469–4472.
- Streitwieser A, Jr, Jagow RH, Suzuki S. J Am Chem Soc. 1958;80:2326–2332.
-
- Hess BA, Jr, Schaad LJ, Pancir J. J Am Chem Soc. 1985;107:149–154.
- Baldwin JE, Reddy VP, Hess BA, Jr, Schaad LJ. J Am Chem Soc. 1988;110:8554–8555.
-
- Lu D-h, Maurice D, Truhlar DG. J Am Chem Soc. 1990;112:6206–6214.
-
- Beno BR, Houk KN, Singleton DA. J Am Chem Soc. 1996;118:9984–9985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

