Descending control of nociception: Specificity, recruitment and plasticity
- PMID: 19146877
- PMCID: PMC2894733
- DOI: 10.1016/j.brainresrev.2008.12.009
Descending control of nociception: Specificity, recruitment and plasticity
Abstract
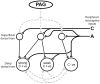
The dorsal horn of the spinal cord is the location of the first synapse in pain pathways, and as such, offers a very powerful target for regulation of nociceptive transmission by both local segmental and supraspinal mechanisms. Descending control of spinal nociception originates from many brain regions and plays a critical role in determining the experience of both acute and chronic pain. The earlier concept of descending control as an "analgesia system" is now being replaced with a more nuanced model in which pain input is prioritized relative to other competing behavioral needs and homeostatic demands. Descending control arises from a number of supraspinal sites, including the midline periaqueductal gray-rostral ventromedial medulla (PAG-RVM) system, and the more lateral and caudal dorsal reticular nucleus (DRt) and ventrolateral medulla (VLM). Inhibitory control from the PAG-RVM system preferentially suppresses nociceptive inputs mediated by C-fibers, preserving sensory-discriminative information conveyed by more rapidly conducting A-fibers. Analysis of the circuitry within the RVM reveals that the neural basis for bidirectional control from the midline system is two populations of neurons, ON-cells and OFF-cells, that are differentially recruited by higher structures important in fear, illness and psychological stress to enhance or inhibit pain. Dynamic shifts in the balance between pain inhibiting and facilitating outflows from the brainstem play a role in setting the gain of nociceptive processing as dictated by behavioral priorities, but are also likely to contribute to pathological pain states.
Figures
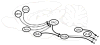
References
-
- Andrew D, Craig AD. Responses of spinothalamic lamina I neurons to maintained noxious mechanical stimulation in the cat. J Neurophysiol. 2002;87:1889–1901. - PubMed
-
- Azami J, Green DL, Roberts MH, Monhemius R. The behavioural importance of dynamically activated descending inhibition from the nucleus reticularis gigantocellularis pars alpha. Pain. 2001;92:53–62. - PubMed
-
- Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–10. - PubMed
-
- Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

