The heterogeneity of human antibody responses to vaccinia virus revealed through use of focused protein arrays
- PMID: 19146908
- PMCID: PMC2673137
- DOI: 10.1016/j.vaccine.2008.12.035
The heterogeneity of human antibody responses to vaccinia virus revealed through use of focused protein arrays
Abstract
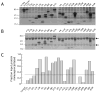
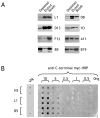
The renewed interest in strategies to combat infectious agents with epidemic potential has led to a re-examination of vaccination protocols against smallpox. To help define which antigens elicit a human antibody response, we have targeted proteins known or predicted to be presented on the surface of the intracellular mature virion (IMV) or the extracellular enveloped virion (EEV). The predicted ectodomains were expressed in a mammalian in vitro coupled transcription/translation reaction using tRNA(lys) precharged with lysine-epsilon-biotin followed by solid phase immobilization on 384-well neutravidin-coated plates. The generated array is highly specific and sensitive in a micro-ELISA format. By comparison of binding of vaccinia-immune sera to the reticulocyte lysate-produced proteins and to secreted post-translationally modified proteins, we demonstrate that for several proteins including the EEV proteins B5 and A33, proper recognition is dependent upon appropriate folding, with little dependence upon glycosylation per se. We further demonstrate that the humoral immune response to vaccinia among different individuals is not uniform in specificity or strength, as different IMV and EEV targets predominate within the group of immunogenic proteins. This heterogeneity likely results from the diversity of HLA Class II alleles and CD4 T helper cell epitopes stimulating B cell antibody production. Our findings have important implications both for design of new recombinant subunit vaccines as well as for methods of assaying the human antibody response utilizing recombinant proteins produced in vitro.
Figures






References
-
- Fauci AS. Emerging infectious diseases: a clear and present danger to humanity. JAMA. 2004 Oct 20;292(15):1887–8. - PubMed
-
- Lane JM, Goldstein J. Evaluation of 21st-century risks of smallpox vaccination and policy options. Ann Intern Med. 2003 Mar 18;138(6):488–93. - PubMed
-
- Rotz LD, Dotson DA, Damon IK, Becher JA. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR Recomm Rep. 2001 Jun 22;50(RR10):1–25. quiz CE1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

