The primary cilium at the crossroads of mammalian hedgehog signaling
- PMID: 19147008
- PMCID: PMC2653622
- DOI: 10.1016/S0070-2153(08)00809-0
The primary cilium at the crossroads of mammalian hedgehog signaling
Abstract
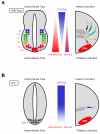
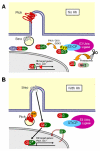
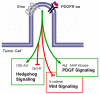
Cilia function as critical sensors of extracellular information, and ciliary dysfunction underlies diverse human disorders including situs inversus, polycystic kidney disease, retinal degeneration, and Bardet-Biedl syndrome. Importantly, mammalian primary cilia have recently been shown to mediate transduction of Hedgehog (Hh) signals, which are involved in a variety of developmental processes. Mutations in several ciliary components disrupt the patterning of the neural tube and limb bud, tissues that rely on precisely coordinated gradients of Hh signal transduction. Numerous components of the Hh pathway, including Patched, Smoothened, and the Gli transcription factors, are present within primary cilia, indicating that key steps of Hh signaling may occur within the cilium. Because dysregulated Hh signaling promotes the development of a variety of human tumors, cilia may also have roles in cancer. Together, these findings have shed light on one mechanism by which primary cilia transduce signals critical for both development and disease.
Figures


References
-
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. - PubMed
-
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
-
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. - PubMed
-
- Arima T, Shibata Y, Yamamoto T. A deep-etching study of the guinea pig tracheal cilium with special reference to the ciliary transitional region. J Ultrastruct Res. 1984;89:34–41. - PubMed
-
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

