Modification of the furan ring of salvinorin A: identification of a selective partial agonist at the kappa opioid receptor
- PMID: 19147366
- PMCID: PMC2680211
- DOI: 10.1016/j.bmc.2008.12.012
Modification of the furan ring of salvinorin A: identification of a selective partial agonist at the kappa opioid receptor
Abstract
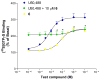
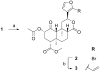
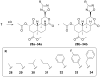
In an effort to find novel agents which selectively target the kappa opioid receptor (KOPR), we modified the furan ring of the highly potent and selective KOPR agonist salvinorin A. Introduction of small substituents at C-16 was well tolerated. 12-epi-Salvinorin A, synthesized in four steps from salvinorin A, was a selective partial agonist at the KOPR. No clear SAR patterns were observed for C-13 aryl ketones. Introducing a hydroxymethylene group between C-12 and the furan ring was tolerated. Small C-13 esters and ethers gave weak KOPR agonists, while all C-13 amides were inactive. Finally, substitution of oxadiazoles for the furan ring abolished affinity for the KOPR. None of the compounds displayed any KOPR antagonism or any affinity for mu or delta opioid receptors.
Figures





References
-
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr J Pharmacol Exp Ther. 2003;305:323. - PubMed
-
- Nestler EJ, Carlezon WA., Jr Biol Psychiatry. 2006;59:1151. - PubMed
-
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr J Pharmacol Exp Ther. 2007;323:838. - PubMed
-
- Portoghese PS, Lipkowski AW, Takemori AE. Life Sci. 1987;40:1287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

