The C-terminal region of laminin beta chains modulates the integrin binding affinities of laminins
- PMID: 19147489
- PMCID: PMC2658076
- DOI: 10.1074/jbc.M809332200
The C-terminal region of laminin beta chains modulates the integrin binding affinities of laminins
Abstract
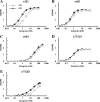
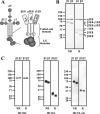
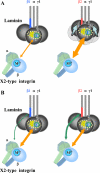
Laminins are major cell-adhesive proteins in basement membranes that are capable of binding to integrins. Laminins consist of three chains (alpha, beta, and gamma), in which three laminin globular modules in the alpha chain and the Glu residue in the C-terminal tail of the gamma chain have been shown to be prerequisites for binding to integrins. However, it remains unknown whether any part of the beta chain is involved in laminin-integrin interactions. We compared the binding affinities of pairs of laminin isoforms containing the beta1 or beta2 chain toward a panel of laminin-binding integrins, and we found that beta2 chain-containing laminins (beta2-laminins) bound more avidly to alpha3beta1 and alpha7X2beta1 integrins than beta1 chain-containing laminins (beta1-laminins), whereas alpha6beta1, alpha6beta4, and alpha7X1beta1 integrins did not show any preference toward beta2-laminins. Because alpha3beta1 contains the "X2-type" variable region in the alpha3 subunit and alpha6beta1 and alpha6beta4 contain the "X1-type" region in the alpha6 subunit, we hypothesized that only integrins containing the X2-type region were capable of discriminating between beta1-laminins and beta2-laminins. In support of this possibility, a putative X2-type variant of alpha6beta1 was produced and found to bind preferentially to beta2-laminins. Production of a series of swap mutants between the beta1 and beta2 chains revealed that the C-terminal 20 amino acids in the coiled-coil domain were responsible for the enhanced integrin binding by beta2-laminins. Taken together, the results provide evidence that the C-terminal region of beta chains is involved in laminin recognition by integrins and modulates the binding affinities of laminins toward X2-type integrins.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

