Activation of TonEBP by calcium controls {beta}1,3-glucuronosyltransferase-I expression, a key regulator of glycosaminoglycan synthesis in cells of the intervertebral disc
- PMID: 19147493
- PMCID: PMC2665104
- DOI: 10.1074/jbc.M807081200
Activation of TonEBP by calcium controls {beta}1,3-glucuronosyltransferase-I expression, a key regulator of glycosaminoglycan synthesis in cells of the intervertebral disc
Abstract
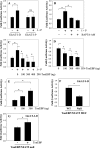
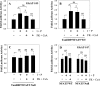
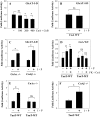
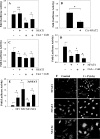
The goal of this investigation was to study the expression and regulation of beta1,3-Glucuronosyltransferase-I (GlcAT-I), a key enzyme regulating GAG synthesis in cells of the intervertebral disc. There was a robust expression of GlcAT-I in the nucleus pulposus in vivo. Treatment with the calcium ionophore ionomycin resulted in increased GlcAT-I expression, whereas GlcAT-I promoter constructs lacking TonE site or a mutant TonE were unresponsive to the ionophore. Experiments using TonEBP and DN-TonEBP constructs showed that TonEBP positively regulated GlcAT-I promoter activity. ChIP analysis confirmed binding of TonEBP to the promoter. We further validated the role of TonEBP in controlling GlcAT-I expression using mouse embryo fibroblasts from TonEBP null mice. GlcAT-I promoter activity in null cells was significantly lower than the wild type cells. In contrast to wild type cells, treatment with ionomycin failed to increase GlcAT-I promoter activity in null cells. We then investigated if calcineurin (Cn)-NFAT signaling played a regulatory role in GlcAT-I expression. Inhibition of Cn following ionomycin treatment did not block GlcAT-I and tauT, a TonEBP-responsive reporter activity. GlcAT-I promoter activity was suppressed by co-expression of Cn, NFAT2, NFAT3, and NFAT4. Moreover, following ionomycin treatment, fibroblasts from CnAalpha and CnAbeta null mice exhibited robust induction in GlcAT-I promoter activity compared with wild type cells. Results of these studies demonstrate that calcium regulates GlcAT-I expression in cells of the nucleus pulposus through a signaling network comprising both activator and suppressor molecules. The results suggest that by controlling both GAG and aggrecan synthesis, disc cells can autoregulate their osmotic environment and accommodate mechanical loading.
Figures




Similar articles
-
BMP-2 and TGF-beta stimulate expression of beta1,3-glucuronosyl transferase 1 (GlcAT-1) in nucleus pulposus cells through AP1, TonEBP, and Sp1: role of MAPKs.J Bone Miner Res. 2010 May;25(5):1179-90. doi: 10.1359/jbmr.091202. J Bone Miner Res. 2010. PMID: 19961337 Free PMC article.
-
Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc.J Bone Miner Res. 2009 Jun;24(6):992-1001. doi: 10.1359/jbmr.090103. J Bone Miner Res. 2009. PMID: 19138132 Free PMC article.
-
TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc.J Biol Chem. 2006 Sep 1;281(35):25416-24. doi: 10.1074/jbc.M601969200. Epub 2006 Jun 13. J Biol Chem. 2006. PMID: 16772300
-
Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP.Matrix Biol. 2014 Nov;40:10-6. doi: 10.1016/j.matbio.2014.08.014. Epub 2014 Aug 27. Matrix Biol. 2014. PMID: 25172826 Free PMC article. Review.
-
Glycosaminoglycan synthesis in the nucleus pulposus: Dysregulation and the pathogenesis of disc degeneration.Matrix Biol. 2018 Oct;71-72:368-379. doi: 10.1016/j.matbio.2018.02.025. Epub 2018 Mar 1. Matrix Biol. 2018. PMID: 29501510 Free PMC article. Review.
Cited by
-
Spatiotemporal diversity and regulation of glycosaminoglycans in cell homeostasis and human disease.Am J Physiol Cell Physiol. 2022 May 1;322(5):C849-C864. doi: 10.1152/ajpcell.00085.2022. Epub 2022 Mar 16. Am J Physiol Cell Physiol. 2022. PMID: 35294848 Free PMC article. Review.
-
Formation, function, and exhaustion of notochordal cytoplasmic vacuoles within intervertebral disc: current understanding and speculation.Oncotarget. 2017 May 23;8(34):57800-57812. doi: 10.18632/oncotarget.18101. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915712 Free PMC article. Review.
-
RNA binding protein HuR regulates extracellular matrix gene expression and pH homeostasis independent of controlling HIF-1α signaling in nucleus pulposus cells.Matrix Biol. 2019 Apr;77:23-40. doi: 10.1016/j.matbio.2018.08.003. Epub 2018 Aug 7. Matrix Biol. 2019. PMID: 30092282 Free PMC article.
-
Hypoxic regulation of β-1,3-glucuronyltransferase 1 expression in nucleus pulposus cells of the rat intervertebral disc: role of hypoxia-inducible factor proteins.Arthritis Rheum. 2011 Jul;63(7):1950-60. doi: 10.1002/art.30342. Arthritis Rheum. 2011. PMID: 21400481 Free PMC article.
-
Role of NFAT5 in inflammatory disorders associated with osmotic stress.Curr Genomics. 2010 Dec;11(8):584-90. doi: 10.2174/138920210793360961. Curr Genomics. 2010. PMID: 21629436 Free PMC article.
References
-
- Feng, H., Danfelter, M., Strömqvist, B., and Heinegård, D. (2006) J. Bone Joint Surg. Am. 88 25–29 - PubMed
-
- Setton, L. A., and Chen, J. (2006) J. Bone Joint Surg. Am. 88 52–57 - PubMed
-
- Ng, L., Grodzinsky, A. J., Patwari, P., Sandy, J., Plaas, A., and Ortiz, C. (2003) J. Struct. Biol. 143 242–257 - PubMed
-
- Tsai, T. T., Danielson, K. G., Guttapalli, A., Oguz, E., Albert, T. J., Shapiro, I. M., and Risbud, M. V. (2006) J. Biol. Chem. 281 25416–25424 - PubMed
-
- Kitagawa, H., Ujikawa, M., and Sugahara, K. (1996) J. Biol. Chem. 271 6583–6585 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

