S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: IMPLICATIONS FOR ASTROCYTE DEVELOPMENT, ACTIVATION, AND TUMOR GROWTH
- PMID: 19147496
- PMCID: PMC2659238
- DOI: 10.1074/jbc.M805897200
S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: IMPLICATIONS FOR ASTROCYTE DEVELOPMENT, ACTIVATION, AND TUMOR GROWTH
Abstract
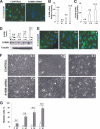
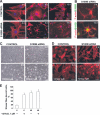
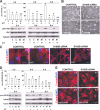
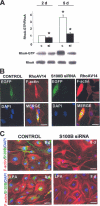
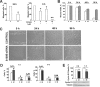
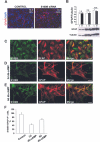
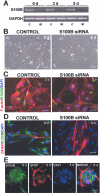
S100B is a Ca(2+)-binding protein of the EF-hand type that is abundantly expressed in astrocytes and has been implicated in the regulation of several intracellular activities, including proliferation and differentiation. We show here that reducing S100B levels in the astrocytoma cell line GL15 and the Müller cell line MIO-M1 by small interference RNA technique results in a rapid disassembly of stress fibers, collapse of F-actin onto the plasma membrane and reduced migration, and acquisition of a stellate shape. Also, S100B-silenced GL15 and MIO-M1 Müller cells show a higher abundance of glial fibrillary acidic protein filaments, which mark differentiated astrocytes, compared with control cells. These effects are dependent on reduced activation of the phosphatidylinositol 3-kinase (PI3K) downstream effectors, Akt and RhoA, and consequently elevated activity of GSK3beta and Rac1 and decreased activity of the RhoA-associated kinase. Also, rat primary astrocytes transiently down-regulate S100B expression when exposed to the differentiating agent dibutyryl cyclic AMP and re-express S100B at later stages of dibutyryl cyclic AMP-induced differentiation. Moreover, reducing S100B levels results in a remarkably slow resumption of S100B expression, suggesting the S100B might regulate its own expression. Finally, we show that S100B interacts with Src kinase, thereby stimulating the PI3K/Akt and PI3K/RhoA pathways. These results suggest that S100B might contribute to reduce the differentiation potential of cells of the astrocytic lineage and participate in the astrocyte activation process in the case of brain insult and in invasive properties of glioma cells.
Figures

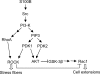
References
-
- Donato, R. (2001) Int. J. Biochem. Cell Biol. 33 637-668 - PubMed
-
- Heizmann, C. W., Fritz, G., and Schaefer, B. W. (2002) Front. Biosci. 7 1356-1368 - PubMed
-
- Marenholz, I., Heizmann, C. W., and Fritz, G. (2004) Biochem. Biophys. Res. Commun. 322 1111-1122 - PubMed
-
- Markowitz, J., MacKerell, A. D., Jr., and Weber, D. J. (2007) Mini Rev. Med. Chem. 7 609-616 - PubMed
-
- Rustandi, R. R., Baldisseri, D. M., and Weber, D. J. (2000) Nat. Struct. Biol. 7 570-574 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

