Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases
- PMID: 19147611
- PMCID: PMC2770105
- DOI: 10.1136/ard.2008.102103
Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases
Abstract
Objectives: Clinical trials of tumour necrosis factor antagonists have raised questions about the potential risk of certain serious adverse events (SAE). To assess the safety of adalimumab in rheumatoid arthritis (RA) over time and across five other immune-mediated inflammatory diseases and to compare adalimumab malignancy and mortality rates with data on the general population.
Methods: This analysis included 19,041 patients exposed to adalimumab in 36 global clinical trials in RA, psoriatic arthritis (PsA), ankylosing spondylitis (AS), Crohn's disease (CD), psoriasis and juvenile idiopathic arthritis (JIA) to 15 April 2007. Events per 100 patient-years were calculated using SAE reported after the first dose to 70 days after the last dose. Standardised incidence rates were calculated for malignancies using national and state-specific databases. Standardised mortality rates (SMR) were calculated for each disease using data from the World Health Organization.
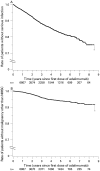
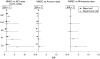
Results: Cumulative rates of SAE of interest in RA have remained stable over time. Rates of SAE of interest for PsA, AS, CD, psoriasis and JIA were similar to or lower than rates for RA. Overall malignancy rates for adalimumab-treated patients were as expected for the general population. SMR across all six diseases indicated that no more deaths occurred with adalimumab than expected in the general population.
Conclusions: Based on 10 years of clinical trial experience across six diseases, this safety report and the established efficacy of adalimumab in these diseases provide the foundation for a better understanding of its benefit-risk profile.
Conflict of interest statement
Competing interests: JP and ALP are employees of Abbott Laboratories. GRB has served as a consultant with Abbott Laboratories, Essex/Schering-Plough, Novartis, Roche and has received grants from Abbott Laboratories, Essex/Schering-Plough, Novartis, Roche, Wyeth and honoraria from Abbott Laboratories, Essex/Schering-Plough, Novartis, Roche and Wyeth. BACD has served as a consultant with Abbott, Centocor, Schering-Plough and has received grants from Abbott, Centocor, Schering-Plough and received honoraria from Abbott, Centocor and Schering-Plough. KG has served as a consultant with and has received grants and honoraria from Abbott Laboratories. DL has served as a consultant with Abbott Laboratories, Amgen, Centocor, Hoffmann-La Roche, Novartis, Pfizer, Regeneron, Xoma and has received grants from Abbott Laboratories, Amgen, Bristol-Myers Squibb, Centocor, Hoffmann-La Roche, Regeneron, Roche and honoraria from Wyeth Pharmaceuticals. RP has served as a consultant with Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Centocor, Elan, Ferring, GlaxoSmithKline, Procter and Gamble, Schering-Plough, Shire, UCB and has received grants from Abbott Laboratories, Axcan, Bristol-Myers Squibb, Centocor, Elan, Millennium, Procter and Gamble and has received honoraria from Abbott, Astra Zeneca, Byk Solvay, Centocor, Elan, Janssen, Procter and Gamble, Prometheus, Schering-Plough and Shire. PM has received grants and honoraria from Abbott Laboratories.
Figures


References
-
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9 - PubMed
-
- van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582–91 - PubMed
-
- van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46 - PubMed
-
- Davis JC, van der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis. Arthritis Rheum 2003;48:3230–6 - PubMed
-
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349:2014–22 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

