agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides
- PMID: 19147840
- PMCID: PMC2633565
- DOI: 10.1073/pnas.0807760106
agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides
Abstract
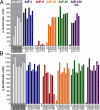
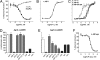
Through the agr quorum-sensing system, staphylococci secrete unique autoinducing peptides (AIPs) and detect their concentration via the AgrC transmembrane receptor, coordinating local bacterial population density with global changes in gene expression. Unique AIP and AgrC variants exist within and between species, and although autologous interactions lead to agr activation, heterologous interactions usually lead to cross-inhibition, resulting in natural quorum-sensing interference. To gain insight into the mechanisms responsible for these phenomena at the level of the receptor, we used random mutagenesis to isolate variants of Staphylococcus aureus AgrC-I with constitutive activity. Constitutive mutations in the sensor domain of the receptor were localized to the last transmembrane helix, whereas those in the histidine kinase domain were mostly clustered to a region near the phosphorylation site histidine. Analysis of these mutants with a range of noncognate AIPs revealed that inhibition is manifested by inverse agonism in certain heterologous pairings and by neutral antagonism in others. In addition, we isolated and characterized an AgrC sensor domain mutant with dramatically broadened activation specificity and reduced sensitivity to inhibition, identifying a single amino acid as a critical determinant of ligand-mediated inhibition. These results suggest that certain noncognate AIPs stabilize an inhibitory receptor conformation that may be a critical feature of the ligand-receptor interaction not initially appreciated in previous analyses of agr inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

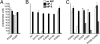
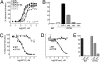
References
-
- Lyon GJ, Novick RP. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides. 2004;25:1389–1403. - PubMed
-
- Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. - PubMed
-
- McDowell P, et al. Structure, activity and evolution of the group I thiolactone peptide quorum-sensing system of Staphylococcus aureus. Mol Microbiol. 2001;41:503–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

