Pyroptosis: host cell death and inflammation
- PMID: 19148178
- PMCID: PMC2910423
- DOI: 10.1038/nrmicro2070
Pyroptosis: host cell death and inflammation
Abstract
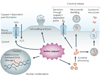
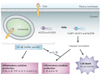
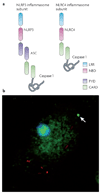
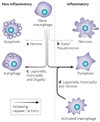
Eukaryotic cells can initiate several distinct programmes of self-destruction, and the nature of the cell death process (non-inflammatory or proinflammatory) instructs responses of neighbouring cells, which in turn dictates important systemic physiological outcomes. Pyroptosis, or caspase 1-dependent cell death, is inherently inflammatory, is triggered by various pathological stimuli, such as stroke, heart attack or cancer, and is crucial for controlling microbial infections. Pathogens have evolved mechanisms to inhibit pyroptosis, enhancing their ability to persist and cause disease. Ultimately, there is a competition between host and pathogen to regulate pyroptosis, and the outcome dictates life or death of the host.
Figures

References
-
- Samali A, Zhivotovsky B, Jones D, Nagata S, Orrenius S. Apoptosis: cell death defined by caspase activation. Cell Death Differ. 1999;6:495–496. - PubMed
-
- Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nature Rev. Immunol. 2004;4:223–231. - PubMed
-
- Frantz S, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J. Mol. Cell. Cardiol. 2003;35:685–694. - PubMed
-
- Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J. Clin. Immunol. 1999;19:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

