The molecular basis of TCR germline bias for MHC is surprisingly simple
- PMID: 19148199
- PMCID: PMC3982143
- DOI: 10.1038/ni.f.219
The molecular basis of TCR germline bias for MHC is surprisingly simple
Abstract
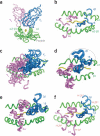
The elusive etiology of germline bias of the T cell receptor (TCR) for major histocompatibility complex (MHC) has been clarified by recent 'proof-of-concept' structural results demonstrating the conservation of specific TCR-MHC interfacial contacts in complexes bearing common variable segments and MHC allotypes. We suggest that each TCR variable-region gene product engages each type of MHC through a 'menu' of structurally coded recognition motifs that have arisen through coevolution. The requirement for MHC-restricted T cell recognition during thymic selection and peripheral surveillance has necessitated the existence of such a coded recognition system. Given these findings, a reconsideration of the TCR-peptide-MHC structural database shows that not only have the answers been there all along but also they were predictable by the first principles of physical chemistry.
Figures
References
-
- Rudolph MG, stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006;24:419–466. - PubMed
-
- Buslepp J, Wang H, Biddison WE, Appella E, Collins EJ. A correlation between TCR Vα docking on MHC and CD8 dependence: implications for T cell selection. Immunity. 2003;19:595–606. - PubMed
-
- Collins EJ, Riddle DS. TCR-MHC docking orientation: natural selection, or thymic selection? Immunol. Res. 2008;41:267–294. - PubMed
-
- Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat. Rev. Immunol. 2006;6:883–894. - PubMed
-
- Tynan FE, et al. T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat. Immunol. 2005;6:1114–1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

