Suppressive effect of hydroquinone, a benzene metabolite, on in vitro inflammatory responses mediated by macrophages, monocytes, and lymphocytes
- PMID: 19148301
- PMCID: PMC2625402
- DOI: 10.1155/2008/298010
Suppressive effect of hydroquinone, a benzene metabolite, on in vitro inflammatory responses mediated by macrophages, monocytes, and lymphocytes
Abstract
We investigated the inhibitory effects of hydroquinone on cytokine release, phagocytosis, NO production, ROS generation, cell-cell/cell fibronectin adhesion, and lymphocyte proliferation. We found that hydroquinone suppressed the production of proinflammatory cytokines [tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and IL-6], secretion of toxic molecules [nitric oxide (NO) and reactive oxygen species (ROS)], phagocytic uptake of FITC-labeled dextran, upregulation of costimulatory molecules, U937 cell-cell adhesion induced by CD18 and CD29, and the proliferation of lymphocytes from the bone marrow and spleen. Considering that (1) environmental chemical stressors reduce the immune response of chronic cigarette smokers and children against bacterial and viral infections and that (2) workers in petroleum factories are at higher risk for cancer, our data suggest that hydroquinone might pathologically inhibit inflammatory responses mediated by monocytes, macrophages, and lymphocytes.
Figures






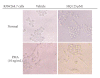




References
-
- Savelkoul HFJ, Neijens HJ. Immune responses during allergic sensitization and the development of atopy. Allergy. 2000;55(11):989–997. - PubMed
-
- Rana SVS, Verma Y. Biochemical toxicity of benzene. Journal of Environmental Biology. 2005;26(2):157–168. - PubMed
-
- Deisinger PJ, Hill TS, English JC. Human exposure to naturally occurring hydroquinone. Journal of Toxicology and Environmental Health. 1996;47(1):31–46. - PubMed
-
- Moerloose KB, Pauwels RA, Joos GF. Short-term cigarette smoke exposure enhances allergic airway inflammation in mice. American Journal of Respiratory and Critical Care Medicine. 2005;172(2):168–172. - PubMed
-
- Kim E, Kang BY, Kim TS. Inhibition of interleukin-12 production in mouse macrophages by hydroquinone, a reactive metabolite of benzene, via suppression of nuclear factor-κB binding activity. Immunology Letters. 2005;99(1):24–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

