Evaluation of polyethylene oxide compacts as gastroretentive delivery systems
- PMID: 19148757
- PMCID: PMC2663672
- DOI: 10.1208/s12249-008-9182-1
Evaluation of polyethylene oxide compacts as gastroretentive delivery systems
Abstract
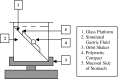
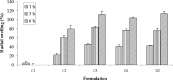
Compacts containing selected bioadhesive polymers, fillers, and binders were investigated for their potential as a bioadhesive gastroretentive delivery system to deliver water soluble and water insoluble compounds in the stomach. Compacts with 90:10, 75:25, and 60:40 of polyvinylpyrrolidone (PVP) and polyethylene oxide (PEO) were evaluated for swelling, dissolution, bioadhesion, and in vitro gastric retention. Compacts containing higher PEO showed higher swelling (111.13%) and bioadhesion (0.62 +/- 0.03 N/cm(2)), and retained their integrity and adherence onto gastric mucosa for about 9 h under in vitro conditions. In vivo gastroretentive property of compacts were evaluated in Yorkshire cross swine. Compacts containing 58% PVP, 40% PEO and 2% of water soluble or water insoluble marker compounds showed gastroadhesive and retentive properties in vivo. It is concluded that PEO in combination with PVP yields a non disintegrating type bioadhesive dosage form which is suitable for gastroretentive applications.
Figures
Similar articles
-
Floating-bioadhesive gastroretentive Caesalpinia pulcherrima-based beads of amoxicillin trihydrate for Helicobacter pylori eradication.Drug Deliv. 2016;23(2):405-19. doi: 10.3109/10717544.2014.916766. Epub 2014 May 28. Drug Deliv. 2016. PMID: 24870198
-
Liquisolid technique for dissolution rate enhancement of a high dose water-insoluble drug (carbamazepine).Int J Pharm. 2007 Aug 16;341(1-2):26-34. doi: 10.1016/j.ijpharm.2007.03.034. Epub 2007 Mar 30. Int J Pharm. 2007. PMID: 17498898
-
Influence of water-soluble polymers on the in vitro performance of floating mucoadhesive tablets containing metformin.Drug Dev Ind Pharm. 2014 Jul;40(7):879-85. doi: 10.3109/03639045.2013.789052. Epub 2013 Apr 22. Drug Dev Ind Pharm. 2014. PMID: 23607725
-
Gastroretentive drug delivery systems.Expert Opin Drug Deliv. 2006 Mar;3(2):217-33. doi: 10.1517/17425247.3.2.217. Expert Opin Drug Deliv. 2006. PMID: 16506949 Review.
-
Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs.Adv Drug Deliv Rev. 2004 May 7;56(9):1273-89. doi: 10.1016/j.addr.2003.12.004. Adv Drug Deliv Rev. 2004. PMID: 15109769 Review.
Cited by
-
Application of design of experiment for polyox and xanthan gum coated floating pulsatile delivery of sumatriptan succinate in migraine treatment.Biomed Res Int. 2014;2014:547212. doi: 10.1155/2014/547212. Epub 2014 Oct 28. Biomed Res Int. 2014. PMID: 25530963 Free PMC article.
-
Novel gastroretentive sustained-release tablet of tacrolimus based on self-microemulsifying mixture: in vitro evaluation and in vivo bioavailability test.Acta Pharmacol Sin. 2011 Oct;32(10):1294-302. doi: 10.1038/aps.2011.90. Epub 2011 Sep 19. Acta Pharmacol Sin. 2011. PMID: 21927013 Free PMC article.
-
Relative bioavailability of chlorothiazide from mucoadhesive compacts in pigs.AAPS PharmSciTech. 2009;10(4):1331-5. doi: 10.1208/s12249-009-9332-0. Epub 2009 Nov 10. AAPS PharmSciTech. 2009. PMID: 19902362 Free PMC article.
-
Montmorillonite-Famotidine/Chitosan Bio-nanocomposite Hydrogels as a Mucoadhesive/Gastroretentive Drug Delivery System.Iran J Pharm Res. 2022 May 12;21(1):e127035. doi: 10.5812/ijpr-127035. eCollection 2022 Dec. Iran J Pharm Res. 2022. PMID: 36060919 Free PMC article.
References
-
- Ali J., et al. Development and evaluation of a gastroretentive drug delivery system for the low-absorption-window drug celecoxib. PDA J. Pharm. Sci. Technol. 2007;61(2):88–96. - PubMed
-
- Basak S. C., Rahman J., Ramalingam M. Design and in vitro testing of a floatable gastroretentive tablet of metformin hydrochloride. Pharmazie. 2007;62(2):145–148. - PubMed
-
- Christensen S. The biological fate of riboflavin in mammals. A survey of literature and own investigations. Acta Pharmacol. Toxicol. (Copenh). 1973;32:3–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources