Advances in oncolytic virus therapy for glioma
- PMID: 19149710
- PMCID: PMC2720101
- DOI: 10.2174/157488909787002573
Advances in oncolytic virus therapy for glioma
Abstract
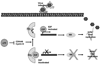
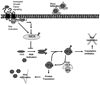
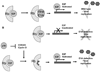
The World Health Organization grossly classifies the various types of astrocytomas using a grade system with grade IV gliomas having the worst prognosis. Oncolytic virus therapy is a novel treatment option for GBM patients. Several patents describe various oncolytic viruses used in preclinical and clinical trials to evaluate safety and efficacy. These viruses are natural or genetically engineered from different viruses such as HSV-1, Adenovirus, Reovirus, and New Castle Disease Virus. While several anecdotal studies have indicated therapeutic advantage, recent clinical trials have revealed the safety of their usage, but demonstration of significant efficacy remains to be established. Oncolytic viruses are being redesigned with an interest in combating the tumor microenvironment in addition to defeating the cancerous cells. Several patents describe the inclusion of tumor microenvironment modulating genes within the viral backbone and in particular those which attack the tumor angiotome. The very innovative approaches being used to improve therapeutic efficacy include: design of viruses which can express cytokines to activate a systemic antitumor immune response, inclusion of angiostatic genes to combat tumor vasculature, and also enzymes capable of digesting tumor extra cellular matrix (ECM) to enhance viral spread through solid tumors. As increasingly more novel viruses are being tested and patented, the future battle against glioma looks promising.
Figures
Similar articles
-
Oncolytic Virotherapy in Glioma Tumors.Int J Mol Sci. 2020 Oct 14;21(20):7604. doi: 10.3390/ijms21207604. Int J Mol Sci. 2020. PMID: 33066689 Free PMC article. Review.
-
Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas.Cell Death Dis. 2020 Jun 25;11(6):485. doi: 10.1038/s41419-020-2696-5. Cell Death Dis. 2020. PMID: 32587256 Free PMC article. Review.
-
Active immunotherapy: oncolytic virus therapy using HSV-1.Adv Exp Med Biol. 2012;746:178-86. doi: 10.1007/978-1-4614-3146-6_14. Adv Exp Med Biol. 2012. PMID: 22639168 Review.
-
[Oncolytic viruses for therapy of malignant glioma].Biomed Khim. 2016 May;62(4):376-90. doi: 10.18097/PBMC20166204376. Biomed Khim. 2016. PMID: 27562991 Review. Russian.
-
Promises of oncolytic viral therapy for adult and children with brain glioma.Curr Opin Oncol. 2023 Nov 1;35(6):529-535. doi: 10.1097/CCO.0000000000000995. Epub 2023 Aug 29. Curr Opin Oncol. 2023. PMID: 37820087 Review.
Cited by
-
Choindroitinase ABC I-mediated enhancement of oncolytic virus spread and anti tumor efficacy: a mathematical model.PLoS One. 2014 Jul 21;9(7):e102499. doi: 10.1371/journal.pone.0102499. eCollection 2014. PLoS One. 2014. PMID: 25047810 Free PMC article.
-
New characteristics of cancer immunotherapy: trends in viral tumor immunotherapy with influenza virus-based approaches.J Zhejiang Univ Sci B. 2025 May 23;26(6):546-556. doi: 10.1631/jzus.B2400381. J Zhejiang Univ Sci B. 2025. PMID: 40571659 Free PMC article. Review.
-
Relapsed and refractory classical Hodgkin lymphoma: could virotherapy help solve the equation?Hum Vaccin Immunother. 2021 Oct 3;17(10):3502-3510. doi: 10.1080/21645515.2021.1924521. Epub 2021 Jun 8. Hum Vaccin Immunother. 2021. PMID: 34101538 Free PMC article. Review.
-
Ral signaling pathway in health and cancer.Cancer Med. 2017 Dec;6(12):2998-3013. doi: 10.1002/cam4.1105. Epub 2017 Oct 18. Cancer Med. 2017. PMID: 29047224 Free PMC article. Review.
-
Impact of tumor microenvironment on oncolytic viral therapy.Cytokine Growth Factor Rev. 2010 Apr-Jun;21(2-3):127-34. doi: 10.1016/j.cytogfr.2010.02.014. Cytokine Growth Factor Rev. 2010. PMID: 20399700 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–117. - PubMed
-
- Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13(8):705–714. - PubMed
-
- Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000;7(10):867–874. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
