Hepatosplenomegaly associated with chronic malaria exposure: evidence for a pro-inflammatory mechanism exacerbated by schistosomiasis
- PMID: 19149774
- PMCID: PMC2680340
- DOI: 10.1111/j.1365-3024.2008.01078.x
Hepatosplenomegaly associated with chronic malaria exposure: evidence for a pro-inflammatory mechanism exacerbated by schistosomiasis
Abstract
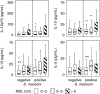
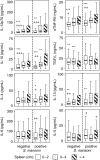
In sub-Saharan Africa, chronic hepatosplenomegaly, with palpable firm/hard organ consistency, is common, particularly among school-aged children. This morbidity can be caused by long-term exposure to malaria, or by Schistosoma mansoni, and it is exacerbated when these two occur together. Although immunological mechanisms probably underlie the pathogenic process, these mechanisms have not been identified, nor is it known whether the two parasites augment the same mechanisms or induce unrelated processes that nonetheless have additive or synergistic effects. Kenyan primary schoolchildren, living in a malaria/schistosomiasis co-transmission area, participated in cross-sectional parasitological and clinical studies in which circulating immune modulator levels were also measured. Plasma IL-12p70, sTNF-RII, IL-10 and IL-13 levels correlated with relative exposure to malaria, and with hepatosplenomegaly. Soluble-TNF-RII and IL-10 were higher in children infected with S. mansoni. Hepatosplenomegaly caused by chronic exposure to malaria was clearly associated with increased circulating levels of pro-inflammatory mediators, with higher levels of regulatory modulators, and with tissue repair cytokines, perhaps being required to control the inflammatory response. The higher levels of regulatory modulators amongst S. mansoni infected children, compared to those without detectable S. mansoni and malarial infections, but exposed to malaria, suggest that S. mansoni infection may augment the underlying inflammatory reaction.
Figures

Similar articles
-
Hepatosplenomegaly in Kenyan schoolchildren: exacerbation by concurrent chronic exposure to malaria and Schistosoma mansoni infection.Trop Med Int Health. 2007 Dec;12(12):1442-9. doi: 10.1111/j.1365-3156.2007.01950.x. Trop Med Int Health. 2007. PMID: 18076550
-
Health implications of chronic hepatosplenomegaly in Kenyan school-aged children chronically exposed to malarial infections and Schistosoma mansoni.Trans R Soc Trop Med Hyg. 2010 Feb;104(2):110-6. doi: 10.1016/j.trstmh.2009.08.006. Epub 2009 Oct 8. Trans R Soc Trop Med Hyg. 2010. PMID: 19818465 Free PMC article.
-
Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection.Infect Immun. 2008 May;76(5):2212-8. doi: 10.1128/IAI.01433-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285496 Free PMC article.
-
Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria.PLoS Negl Trop Dis. 2011 Aug;5(8):e1149. doi: 10.1371/journal.pntd.0001149. Epub 2011 Aug 30. PLoS Negl Trop Dis. 2011. PMID: 21912707 Free PMC article. Review.
-
Haemostatic abnormalities in hepatosplenic schistosomiasis mansoni.Parasitol Int. 2003 Dec;52(4):351-9. doi: 10.1016/s1383-5769(03)00051-5. Parasitol Int. 2003. PMID: 14665393 Review.
Cited by
-
Trichinella spiralis co-infection exacerbates Plasmodium berghei malaria-induced hepatopathy.Parasit Vectors. 2020 Sep 3;13(1):440. doi: 10.1186/s13071-020-04309-6. Parasit Vectors. 2020. PMID: 32883347 Free PMC article.
-
Intestinal schistosomiasis of Ijinga Island, north-western Tanzania: prevalence, intensity of infection, hepatosplenic morbidities and their associated factors.BMC Infect Dis. 2019 Oct 7;19(1):832. doi: 10.1186/s12879-019-4451-z. BMC Infect Dis. 2019. PMID: 31590657 Free PMC article.
-
Helminth parasites alter protection against Plasmodium infection.Biomed Res Int. 2014;2014:913696. doi: 10.1155/2014/913696. Epub 2014 Sep 8. Biomed Res Int. 2014. PMID: 25276830 Free PMC article. Review.
-
Deferiprone-Resveratrol Hybrid, an Iron-Chelating Compound, Acts as an Antimalarial and Hepatoprotective Agent in Plasmodium berghei-Infected Mice.Bioinorg Chem Appl. 2022 Nov 24;2022:3869337. doi: 10.1155/2022/3869337. eCollection 2022. Bioinorg Chem Appl. 2022. PMID: 36466999 Free PMC article.
-
Schistosoma mansoni Infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable?PLoS Negl Trop Dis. 2011 Jan 4;5(1):e938. doi: 10.1371/journal.pntd.0000938. PLoS Negl Trop Dis. 2011. PMID: 21245910 Free PMC article.
References
-
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. - PubMed
-
- Sowunmi A. Hepatomegaly in acute falciparum malaria in children. Trans R Soc Trop Med Hyg. 1996;90:540–542. - PubMed
-
- Sowunmi A, Adedeji AA, Sowunmi CO, et al. Clinical characteristics and disposition kinetics of the hepatomegaly associated with acute, uncomplicated,Plasmodium falciparum malaria in children. Ann Trop Med Parasitol. 2001;95:7–18. - PubMed
-
- Greenwood BM. Asymptomatic malaria infections – do they matter? Parasitol Today. 1987;3:206–214. - PubMed
-
- McGregor IA&, Smith DA. A health, nutrition, and parasitological survey in a rural village (Keneba) in West Kiang, Gambia. Trans R Soc Trop Med Hyg. 1952;46:403. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

