The chromatin remodelling factor BRG1 is a novel binding partner of the tumor suppressor p16INK4a
- PMID: 19149898
- PMCID: PMC2644676
- DOI: 10.1186/1476-4598-8-4
The chromatin remodelling factor BRG1 is a novel binding partner of the tumor suppressor p16INK4a
Abstract
Background: CDKN2A/p16INK4a is frequently altered in human cancers and it is the most important melanoma susceptibility gene identified to date. p16INK4a inhibits pRb phosphorylation and induces cell cycle arrest, which is considered its main tumour suppressor function. Nevertheless, additional activities may contribute to the tumour suppressor role of p16INK4a and could help explain its specific association with melanoma predisposition. To identify such functions we conducted a yeast-two-hybrid screen for novel p16INK4a binding partners.
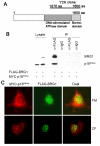
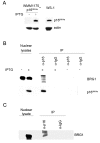
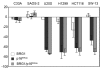
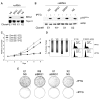
Results: We now report that p16INK4a interacts with the chromatin remodelling factor BRG1. We investigated the cooperative roles of p16INK4a and BRG1 using a panel of cell lines and a melanoma cell model with inducible p16INK4a expression and BRG1 silencing. We found evidence that BRG1 is not required for p16INK4a-induced cell cycle inhibition and propose that the p16INK4a-BRG1 complex regulates BRG1 chromatin remodelling activity. Importantly, we found frequent loss of BRG1 expression in primary and metastatic melanomas, implicating this novel p16INK4a binding partner as an important tumour suppressor in melanoma.
Conclusion: This data adds to the increasing evidence implicating the SWI/SNF chromatin remodelling complex in tumour development and the association of p16INK4a with chromatin remodelling highlights potentially new functions that may be important in melanoma predisposition and chemoresistance.
Figures


References
-
- Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril MF, Azizi E, Bianchi-Scarra G, Bishop DT, Bressac-de Paillerets B, et al. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Res. 2006;66:9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. - DOI - PubMed
-
- Stiegler P, De Luca A, Bagella L, Giordano A. The COOH-terminal region of pRb2/p130 binds to histone deacetylase 1 (HDAC1), enhancing transcriptional repression of the E2F-dependent cyclin A promoter. Cancer Research. 1998;58:5049–5052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

