Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example
- PMID: 19149900
- PMCID: PMC2639595
- DOI: 10.1186/1471-2334-9-2
Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example
Abstract
Background: Our aim was to determine the efficacy of a trivalent inactivated split virus influenza vaccine (TIV) against culture-confirmed influenza A and/or B in adults 18 to 64 years of age during the 2005/2006 season in the Czech Republic.
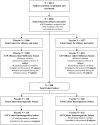
Methods: 6203 subjects were randomized to receive TIV (N = 4137) or placebo (N = 2066). The sample size was based on an assumed attack rate of 4% which provided 90% power to reject the hypothesis that vaccine efficacy (VE) was > or = 45%. Cases of influenza like illness (defined as fever (oral temperature > or =37.8 degrees C) plus cough and/or sore throat) were identified both by active (biweekly phone contact) and passive (self reporting) surveillance and nasal and throat swabs were collected from subjects for viral culture.
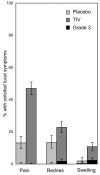
Results: TIV was well tolerated and induced a good immune response. The 2005/2006 influenza season was exceptionally mild in the study area, as it was throughout Europe, and only 46 culture-confirmed cases were found in the study cohort (10 influenza A and 36 influenza B). Furthermore among the B isolates, 35 were identified as B/Hong Kong 330/2001-like (B/Victoria/2/87 lineage) which is antigenically unrelated to the vaccine B strain (B/Yamagata/16/88 lineage). The attack rate in the vaccine group (0.7%) was not statistically significantly different from the attack rate in the placebo group (0.9%).
Conclusion: Due to the atypical nature of the influenza season during this study we were unable to assess TIV efficacy. This experience illustrates the challenge of conducting a prospective influenza vaccine efficacy trial during a single season when influenza attack rates and drift in circulating strains or B virus lineage match can be difficult to estimate in advance.
Trial registration: Clinical trial registery: NCT00197223.
Figures

References
-
- World Health Organization (WHO) Influenza vaccines. Wkly Epidemiol Rec. 2005;80:279–287. - PubMed
-
- Advisory Committee on immunization Practices (ACIP) Recommendations of the Advisory Committee on immunization Practices (ACIP) Prevention and control of influenza. Morbidity and Mortality Weekly Report. 2008;57(RR-7) - PubMed
-
- Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2004. p. CD001269. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

