Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage
- PMID: 19149911
- PMCID: PMC2779114
- DOI: 10.1017/S1461145708009802
Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage
Abstract
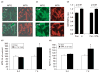
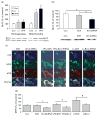
Accumulating evidence suggests that mitochondrial dysfunction plays a critical role in the progression of a variety of neurodegenerative and psychiatric disorders. Thus, enhancing mitochondrial function could potentially help ameliorate the impairments of neural plasticity and cellular resilience associated with a variety of neuropsychiatric disorders. A series of studies was undertaken to investigate the effects of mood stabilizers on mitochondrial function, and against mitochondrially mediated neurotoxicity. We found that long-term treatment with lithium and valproate (VPA) enhanced cell respiration rate. Furthermore, chronic treatment with lithium or VPA enhanced mitochondrial function as determined by mitochondrial membrane potential, and mitochondrial oxidation in SH-SY5Y cells. In-vivo studies showed that long-term treatment with lithium or VPA protected against methamphetamine (Meth)-induced toxicity at the mitochondrial level. Furthermore, these agents prevented the Meth-induced reduction of mitochondrial cytochrome c, the mitochondrial anti-apoptotic Bcl-2/Bax ratio, and mitochondrial cytochrome oxidase (COX) activity. Oligoarray analysis demonstrated that the gene expression of several proteins related to the apoptotic pathway and mitochondrial functions were altered by Meth, and these changes were attenuated by treatment with lithium or VPA. One of the genes, Bcl-2, is a common target for lithium and VPA. Knock-down of Bcl-2 with specific Bcl-2 siRNA reduced the lithium- and VPA-induced increases in mitochondrial oxidation. These findings illustrate that lithium and VPA enhance mitochondrial function and protect against mitochondrially mediated toxicity. These agents may have potential clinical utility in the treatment of other diseases associated with impaired mitochondrial function, such as neurodegenerative diseases and schizophrenia.
Figures
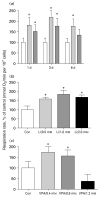
 , lithium;
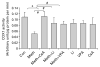
, lithium;  , VPA. SH-SY5Y cells were exposed to lithium (1.2 mM) or VPA (1.0 mM) for 1, 3, or 6 d in serum-free, inositol-free Dulbecco’s Modified Eagle’s Medium. Cellular respiration was determined as described in the Methods section. (b) Dose–response curve for the effect of lithium on cellular respiration. (c) Dose–response curve for the effect of VPA on cellular respiration. Both lithium and VPA increased SH-SY5Y cell respiration rate at therapeutically relevant concentrations. Data are expressed as mean±S.E.M. (one-way ANOVA, N=3, n=5 for each group; Tukey’s multiple comparison test: * p<0.05).
, VPA. SH-SY5Y cells were exposed to lithium (1.2 mM) or VPA (1.0 mM) for 1, 3, or 6 d in serum-free, inositol-free Dulbecco’s Modified Eagle’s Medium. Cellular respiration was determined as described in the Methods section. (b) Dose–response curve for the effect of lithium on cellular respiration. (c) Dose–response curve for the effect of VPA on cellular respiration. Both lithium and VPA increased SH-SY5Y cell respiration rate at therapeutically relevant concentrations. Data are expressed as mean±S.E.M. (one-way ANOVA, N=3, n=5 for each group; Tukey’s multiple comparison test: * p<0.05).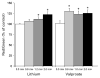
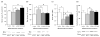

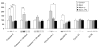
References
-
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Adler CM, DelBello MP, Strakowski SM. Brain network dysfunction in bipolar disorder. CNS Spectrums. 2006;11:312–320. - PubMed
-
- Almeida A, Medina JM. A rapid method for the isolation of metabolically active mitochondria from rat neurons and astrocytes in primary culture. Brain Research. 1998;2:209–214. - PubMed
-
- Bar-Am O, Weinreb O, Amit T, Youdim MB. Regulation of Bcl-2 family proteins, neurotrophic factors, and APP processing in the neurorescue activity of propargylamine. FASEB Journal. 2005;19:1899–1901. - PubMed
-
- Belloc F, Belaud-Rotureau MA, Lavignolle V, Bascans E, Braz-Pereira E, Durrieu F, Lacombe F. Flow cytometry detection of caspase 3 activation in preapoptotic leukemic cells. Cytometry. 2000;40:151–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

