PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination
- PMID: 19150425
- PMCID: PMC2643372
- DOI: 10.1016/j.molcel.2008.11.023
PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination
Abstract
Tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) is a key mediator in TNF signaling. Previous studies suggested that TRAF2 functions as an adaptor in the NF-kappaB and AP-1 pathways. However, the precise molecular mechanisms by which TRAF2 relays signals are unknown. We previously reported that TRAF2 is phosphorylated following TNF stimulation and now identify the PKC kinases responsible for phosphorylation. Phosphorylated TRAF2 facilitates recruitment of IKKalpha and IKKbeta to the TNF receptor. Phosphorylation also determines K63-linked polyubiquitination of TRAF2 at lysine 31. TRAF2 K63-linked ubiquitination contributes to associations with TAB2/3 and activation of the downstream IKK and JNK kinases. The combined data reveal that phosphorylation of TRAF2 plays a critical role in TNF signaling by directing the IKK complex to the membrane, promoting TRAF2 K63-linked ubiquitination, and positioning the IKKalpha and IKKbeta chains with the TAK1/TAB kinase.
Figures

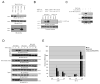
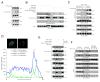
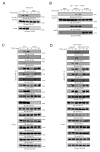

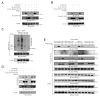

Similar articles
-
Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation.J Biol Chem. 2010 Feb 19;285(8):5347-60. doi: 10.1074/jbc.M109.076976. Epub 2009 Dec 28. J Biol Chem. 2010. PMID: 20038579 Free PMC article.
-
Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2.Nature. 2010 Jun 24;465(7301):1084-8. doi: 10.1038/nature09128. Nature. 2010. PMID: 20577214 Free PMC article.
-
TRAF2 phosphorylation modulates tumor necrosis factor alpha-induced gene expression and cell resistance to apoptosis.Mol Cell Biol. 2009 Jan;29(2):303-14. doi: 10.1128/MCB.00699-08. Epub 2008 Nov 3. Mol Cell Biol. 2009. PMID: 18981220 Free PMC article.
-
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review.
-
TNFR1 signaling kinetics: spatiotemporal control of three phases of IKK activation by posttranslational modification.Cell Signal. 2013 Aug;25(8):1654-64. doi: 10.1016/j.cellsig.2013.04.005. Epub 2013 Apr 21. Cell Signal. 2013. PMID: 23612498 Free PMC article. Review.
Cited by
-
TNF-driven cell fate: till HACE do us part.Oncotarget. 2016 Jul 19;7(29):44871-44872. doi: 10.18632/oncotarget.10168. Oncotarget. 2016. PMID: 27343549 Free PMC article. No abstract available.
-
TRAF2 is an NF-κB-activating oncogene in epithelial cancers.Oncogene. 2015 Jan 8;34(2):209-16. doi: 10.1038/onc.2013.543. Epub 2013 Dec 23. Oncogene. 2015. PMID: 24362534 Free PMC article.
-
NEMO binds ubiquitinated TANK-binding kinase 1 (TBK1) to regulate innate immune responses to RNA viruses.PLoS One. 2012;7(9):e43756. doi: 10.1371/journal.pone.0043756. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028469 Free PMC article.
-
Loss of the HPV-infection resistance EVER2 protein impairs NF-κB signaling pathways in keratinocytes.PLoS One. 2014 Feb 19;9(2):e89479. doi: 10.1371/journal.pone.0089479. eCollection 2014. PLoS One. 2014. PMID: 24586810 Free PMC article.
-
LUBAC regulates NF-κB activation upon genotoxic stress by promoting linear ubiquitination of NEMO.EMBO J. 2011 Aug 2;30(18):3741-53. doi: 10.1038/emboj.2011.264. EMBO J. 2011. PMID: 21811235 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

