Suppression of HIV-1 Nef translation by Sam68 mutant-induced stress granules and nef mRNA sequestration
- PMID: 19150430
- PMCID: PMC2644331
- DOI: 10.1016/j.molcel.2008.11.024
Suppression of HIV-1 Nef translation by Sam68 mutant-induced stress granules and nef mRNA sequestration
Abstract
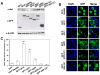
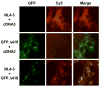
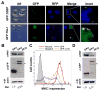
HIV-1 Nef plays important roles in HIV-1 replication and pathogenesis. It is translated from completely spliced HIV-1 RNA, and its expression is inherently regulated at the levels of viral DNA transcription and RNA splicing. Here we show that Sam68 cytoplasmic mutants potently suppress Nef expression. The suppression requires Sam68 domain aa 269-321 and is correlated with its ability to induce stress granules. In addition, the suppression is specific to Nef, and direct binding to nef mRNA 3'UTR confers the suppression specificity. Furthermore, nef mRNA is targeted to and enriched in these induced stress granules. Importantly, Nef suppression occurs in the context of HIV-1 infection of CD4+ T lymphocytes with little MHC I and CD4 downregulation. Taken together, these results demonstrate that stress granule induction and nef mRNA sequestration account for this translational suppression of Nef expression and offer a strategy for development of anti-HIV therapeutics to buttress our fight against HIV/AIDS.
Figures
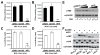


Similar articles
-
Sam68 relocalization into stress granules in response to oxidative stress through complexing with TIA-1.Exp Cell Res. 2009 Nov 15;315(19):3381-95. doi: 10.1016/j.yexcr.2009.07.011. Epub 2009 Jul 14. Exp Cell Res. 2009. PMID: 19615357 Free PMC article.
-
Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility complex antigen expression.J Virol. 2000 Jun;74(12):5691-701. doi: 10.1128/jvi.74.12.5691-5701.2000. J Virol. 2000. PMID: 10823877 Free PMC article.
-
Multifunctional Roles of the N-Terminal Region of HIV-1SF2Nef Are Mediated by Three Independent Protein Interaction Sites.J Virol. 2019 Dec 12;94(1):e01398-19. doi: 10.1128/JVI.01398-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597760 Free PMC article.
-
Association between HIV-1 Nef-mediated MHC-I downregulation and the maintenance of the replication-competent latent viral reservoir in individuals with virally suppressed HIV-1 in Uganda: an exploratory cohort study.Lancet Microbe. 2025 May;6(5):101018. doi: 10.1016/j.lanmic.2024.101018. Epub 2025 Mar 12. Lancet Microbe. 2025. PMID: 40088911 Free PMC article.
-
Intrinsic properties and plasma membrane trafficking route of Src family kinase SH4 domains sensitive to retargeting by HIV-1 Nef.J Biol Chem. 2018 May 18;293(20):7824-7840. doi: 10.1074/jbc.RA118.002794. Epub 2018 Mar 27. J Biol Chem. 2018. PMID: 29588370 Free PMC article.
Cited by
-
MDA5 localizes to stress granules, but this localization is not required for the induction of type I interferon.J Virol. 2013 Jun;87(11):6314-25. doi: 10.1128/JVI.03213-12. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536668 Free PMC article.
-
Sam68/KHDRBS1 is critical for colon tumorigenesis by regulating genotoxic stress-induced NF-κB activation.Elife. 2016 Jul 25;5:e15018. doi: 10.7554/eLife.15018. Elife. 2016. PMID: 27458801 Free PMC article.
-
Analysis of the interaction between host factor Sam68 and viral elements during foot-and-mouth disease virus infections.Virol J. 2015 Dec 23;12:224. doi: 10.1186/s12985-015-0452-8. Virol J. 2015. PMID: 26695943 Free PMC article.
-
Sam68 functions in nuclear export and translation of HIV-1 RNA.RNA Biol. 2009 Sep-Oct;6(4):384-6. doi: 10.4161/rna.6.4.8920. Epub 2009 Sep 1. RNA Biol. 2009. PMID: 19535902 Free PMC article.
-
Herpes simplex virus 2 infection impacts stress granule accumulation.J Virol. 2012 Aug;86(15):8119-30. doi: 10.1128/JVI.00313-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623775 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

