Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats
- PMID: 19150464
- PMCID: PMC2677987
- DOI: 10.1016/j.neuro.2008.12.007
Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats
Abstract
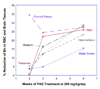
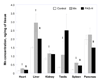
Para-aminosalicylic acid (PAS), an FDA-approved anti-tuberculosis drug, has been used successfully in the treatment of severe manganese (Mn)-induced Parkinsonism in humans [Jiang Y-M, Mo X-A, Du FQ, Fu X, Zhu X-Y, Gao H-Y, et al. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med 2006;48:644-9]. This study was conducted to explore the capability of PAS in reducing Mn concentrations in body fluids and tissues of Mn-exposed animals. Sprague-Dawley rats received daily intraperitoneally (i.p.) injections of 6mg Mn/kg, 5 days/week for 4 weeks, followed by a daily subcutaneously (s.c.) dose of PAS (100 and 200mg/kg as the PAS-L and PAS-H group, respectively) for another 2, 3 or 6 weeks. Mn exposure significantly increased the concentrations of Mn in plasma, red blood cells (RBC), cerebrospinal fluid (CSF), brain and soft tissues. Following PAS-H treatment for 3 weeks, Mn levels in liver, heart, spleen and pancreas were significantly reduced by 25-33%, while 3 weeks of PAS-L treatment did not show any effect. Further therapy with PAS-H for 6 weeks reduced Mn levels in striatum, thalamus, choroid plexus, hippocampus and frontal cortex by 16-29% (p<0.05). Mn exposure greatly increased iron (Fe) and copper (Cu) concentrations in CSF, brain and liver. Treatment with PAS-H restored Fe and Cu levels comparable with control. These data suggest that PAS likely acts as a chelating agent to mobilize and remove tissue Mn. A high-dose and prolonged PAS treatment appears necessary for its therapeutic effectiveness.
Figures
Similar articles
-
Sodium p-Aminosalicylic Acid Reverses Sub-Chronic Manganese-Induced Impairments of Spatial Learning and Memory Abilities in Rats, but Fails to Restore γ-Aminobutyric Acid Levels.Int J Environ Res Public Health. 2017 Apr 10;14(4):400. doi: 10.3390/ijerph14040400. Int J Environ Res Public Health. 2017. PMID: 28394286 Free PMC article.
-
Preventive impacts of PAS-Na on the slow growth and activated inflammatory responses in Mn-exposed rats.J Trace Elem Med Biol. 2019 Jul;54:134-141. doi: 10.1016/j.jtemb.2019.04.013. Epub 2019 Apr 25. J Trace Elem Med Biol. 2019. PMID: 31109603
-
Protective effects of sodium p-aminosalicylic acid on learning and memory via increasing the number of basal forebrain choline acetyltransferase neurons in manganese-exposed rats.Hum Exp Toxicol. 2015 Mar;34(3):240-8. doi: 10.1177/0960327114529454. Epub 2014 Jun 27. Hum Exp Toxicol. 2015. PMID: 24972623
-
Treatment of manganese and lead poisoning with sodium para-aminosalicylic acid: A contemporary update.Toxicol Lett. 2024 Jul;398:69-81. doi: 10.1016/j.toxlet.2024.06.009. Epub 2024 Jun 22. Toxicol Lett. 2024. PMID: 38909920 Review.
-
Manganese and its role in Parkinson's disease: from transport to neuropathology.Neuromolecular Med. 2009;11(4):252-66. doi: 10.1007/s12017-009-8083-0. Neuromolecular Med. 2009. PMID: 19657747 Free PMC article. Review.
Cited by
-
Sodium P-aminosalicylic Acid Attenuates Manganese-Induced Neuroinflammation in BV2 Microglia by Modulating NF-κB Pathway.Biol Trace Elem Res. 2021 Dec;199(12):4688-4699. doi: 10.1007/s12011-021-02581-w. Epub 2021 Jan 14. Biol Trace Elem Res. 2021. PMID: 33447908
-
Mechanisms of manganese-induced neurotoxicity and the pursuit of neurotherapeutic strategies.Front Pharmacol. 2022 Dec 20;13:1011947. doi: 10.3389/fphar.2022.1011947. eCollection 2022. Front Pharmacol. 2022. PMID: 36605395 Free PMC article. Review.
-
Consensus of Expert Opinion for the Diagnosis and Management of Hypermanganesaemia With Dystonia 1 and 2.J Inherit Metab Dis. 2025 May;48(3):e70031. doi: 10.1002/jimd.70031. J Inherit Metab Dis. 2025. PMID: 40320765 Free PMC article.
-
Neuroprotective and Therapeutic Strategies for Manganese-Induced Neurotoxicity.Clin Pharmacol Transl Med. 2017;1(2):54-62. Epub 2017 May 26. Clin Pharmacol Transl Med. 2017. PMID: 30854510 Free PMC article.
-
Subacute manganese exposure in rats is a neurochemical model of early manganese toxicity.Neurotoxicology. 2014 Sep;44:303-13. doi: 10.1016/j.neuro.2014.08.001. Epub 2014 Aug 10. Neurotoxicology. 2014. PMID: 25117542 Free PMC article.
References
-
- Aschner M, Aschner JL. Manganese transport across the blood–brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24:857–860. - PubMed
-
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. - PubMed
-
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. - PubMed
-
- Chandra SV, Shukla GS, Srivastawa RS, Singh H, Gupta VP. An exploratory study of manganese exposure to welders. Clin Toxicol. 1981;18:407–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

