Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2
- PMID: 19150498
- PMCID: PMC2643333
- DOI: 10.1016/j.nbd.2008.12.004
Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2
Abstract
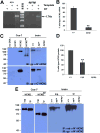
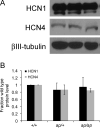
Analysis of naturally occurring mutations that cause seizures in rodents has advanced understanding of the molecular mechanisms underlying epilepsy. Abnormalities of I(h) and h channel expression have been found in many animal models of absence epilepsy. We characterized a novel spontaneous mutant mouse, apathetic (ap/ap), and identified the ap mutation as a 4 base pair insertion within the coding region of Hcn2, the gene encoding the h channel subunit 2 (HCN2). We demonstrated that Hcn2(ap) mRNA is reduced by 90% compared to wild type, and the predicted truncated HCN2(ap) protein is absent from the brain tissue of mice carrying the ap allele. ap/ap mice exhibited ataxia, generalized spike-wave absence seizures, and rare generalized tonic-clonic seizures. ap/+ mice had a normal gait, occasional absence seizures and an increased severity of chemoconvulsant-induced seizures. These findings help elucidate basic mechanisms of absence epilepsy and suggest HCN2 may be a target for therapeutic intervention.
Conflict of interest statement
Figures

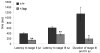


References
-
- Abbas SY, Ying SW, Goldstein PA. Compartmental distribution of hyperpolarization-activated cyclic-nucleotide-gated channel 2 and hyperpolarization-activated cyclic-nucleotide-gated channel 4 in thalamic reticular and thalamocortical relay neurons. Neuroscience. 2006;141:1811–1825. - PubMed
-
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–6836. - PMC - PubMed
-
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. - PMC - PubMed
-
- Browne TR. Ethosuximide (Zarontin) and other succinimides. In: Browne TR, Feldman RG, editors. Epilepsy: Diagnosis and Management. Little Brown & Co; Boston, Mass: 1983. pp. 215–224.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

