Roles of gastric mucin-type O-glycans in the pathogenesis of Helicobacter pylori infection
- PMID: 19150806
- PMCID: PMC2667159
- DOI: 10.1093/glycob/cwp004
Roles of gastric mucin-type O-glycans in the pathogenesis of Helicobacter pylori infection
Abstract
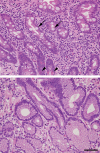
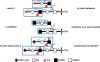
Helicobacter pylori is a Gram-negative bacterium that infects over 50% of the world's population. This organism causes various gastric diseases such as chronic gastritis, peptic ulcer, and gastric cancer. H. pylori possesses lipopolysaccharides that share structural similarity to Lewis blood group antigens in gastric mucosa. Such antigenic mimicry could result in immune tolerance against antigens of this pathogen. On the other hand, H. pylori colonizes gastric mucosa by utilizing adhesins that bind Lewis blood group antigen-related carbohydrates expressed on gastric epithelial cells. After colonization, H. pylori induces acute inflammatory responses mainly by neutrophils. This acute phase is gradually replaced by a chronic inflammatory response. In chronic gastritis, lymphocytes infiltrate the lamina propria, and such infiltration is facilitated by the interaction between L-selectin on lymphocytes and peripheral lymph node addressin (PNAd), which contains 6-sulfo sialyl Lewis X-capped O-glycans, on high endothelial venule (HEV)-like vessels. H. pylori barely colonizes gland mucous cell-derived mucin where alpha1,4-GlcNAc-capped O-glycans exist. In vitro experiments show that alpha1,4-GlcNAc-capped O-glycans function as a natural antibiotic to inhibit H. pylori growth. These findings show that distinct sets of carbohydrates expressed in the stomach are closely associated with pathogenesis and prevention of H. pylori-related diseases, providing therapeutic potentialities based on specific carbohydrate modulation.
Figures


References
-
- Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. - PubMed
-
- Appelmelk BJ, Monteiro MA, Martin SL, Moran AP, Vandenbroucke- Grauls CM. Why Helicobacter pylori has Lewis antigens? Trends Microbiol. 2000;8:565–570. - PubMed
-
- Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikstrom S, Sjostrom R, Linden S, Backstrom A, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. - PubMed
-
- Catrenich CE, Chestnut MH. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

