Polymerization of actin does not regulate desensitization in human basophils
- PMID: 19150851
- PMCID: PMC2656430
- DOI: 10.1189/jlb.1008668
Polymerization of actin does not regulate desensitization in human basophils
Abstract
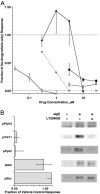
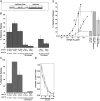
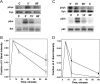
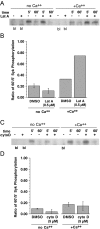
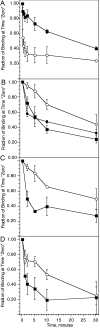
Previous studies have suggested that maintenance of IgE-mediated signaling results from regulation of the activity of signaling complexes by actin polymerization. This process is also hypothesized to be related to desensitization of basophils and mast cells. Recent studies demonstrated that any signaling process dependent on syk or PI-3K activity cannot be a mechanism of desensitization, and in this context, syk and PI-3K inhibitors were found to inhibit actin polymerization. Inhibitors of actin polymerization were tested for their effect on desensitization of human peripheral blood basophils. Latrunculin A, in particular, removed all resting and stimulated f-actin but did not inhibit desensitization. Cytochalasin D and latrunculin A also did not reverse the loss of syk phosphorylation that accompanies desensitization. These results demonstrate that desensitization mechanisms are not dependent on actin polymerization. In this context, it was also shown that progressive immobilization of Fc epsilon RI during aggregation was sensitive to syk or actin polymerization inhibition. Therefore, desensitization is also not dependent on receptor immobilization. These studies demonstrate that desensitization is not the result of two signaling pathways once considered relevant to down-regulation of IgE-mediated signaling.
Figures
Similar articles
-
Actin cytoskeleton-dependent down-regulation of early IgE-mediated signaling in human basophils.J Leukoc Biol. 2004 May;75(5):928-37. doi: 10.1189/jlb.0903431. Epub 2004 Mar 2. J Leukoc Biol. 2004. PMID: 14996825
-
Involvement of filamentous actin in setting the threshold for degranulation in mast cells.Eur J Immunol. 2004 Jun;34(6):1627-36. doi: 10.1002/eji.200424991. Eur J Immunol. 2004. PMID: 15162432
-
The influence of actin microfilaments on signaling by the receptor with high-affinity for IgE.Mol Immunol. 2004 Jul;41(8):817-29. doi: 10.1016/j.molimm.2004.03.033. Mol Immunol. 2004. PMID: 15234561
-
A current understanding of Fc epsilon RI-dependent mast cell activation.Curr Allergy Asthma Rep. 2008 Mar;8(1):14-20. doi: 10.1007/s11882-008-0004-z. Curr Allergy Asthma Rep. 2008. PMID: 18377769 Review.
-
Fc(epsilon)Ri-dependent signaling pathways in human mast cells.Clin Immunol. 2001 May;99(2):198-210. doi: 10.1006/clim.2001.4992. Clin Immunol. 2001. PMID: 11318592 Review. No abstract available.
Cited by
-
Optimizing drug inhibition of IgE-mediated anaphylaxis in mice.J Allergy Clin Immunol. 2022 Feb;149(2):671-684.e9. doi: 10.1016/j.jaci.2021.06.022. Epub 2021 Jun 26. J Allergy Clin Immunol. 2022. PMID: 34186142 Free PMC article.
-
Rapid desensitization of humanized mice with anti-human FcεRIα monoclonal antibodies.J Allergy Clin Immunol. 2020 Mar;145(3):907-921.e3. doi: 10.1016/j.jaci.2019.12.003. Epub 2019 Dec 11. J Allergy Clin Immunol. 2020. PMID: 31836406 Free PMC article.
-
Marked differences in the signaling requirements for expression of CD203c and CD11b versus CD63 expression and histamine release in human basophils.Int Arch Allergy Immunol. 2012;159(3):243-52. doi: 10.1159/000332150. Epub 2012 Jun 20. Int Arch Allergy Immunol. 2012. PMID: 22722613 Free PMC article.
-
Immunology in the Clinic Review Series; focus on allergies: basophils as biomarkers for assessing immune modulation.Clin Exp Immunol. 2012 Jan;167(1):59-66. doi: 10.1111/j.1365-2249.2011.04503.x. Clin Exp Immunol. 2012. PMID: 22132885 Free PMC article. Review.
-
Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcεRI expression characterized by other methods of desensitization.Clin Exp Allergy. 2012 Jul;42(7):1060-70. doi: 10.1111/j.1365-2222.2012.04013.x. Clin Exp Allergy. 2012. PMID: 22702505 Free PMC article.
References
-
- MacGlashan D W., Jr Two regions of down-regulation in the IgE-mediated signaling pathway in human basophils. J Immunol. 2003;170:4914–4925. - PubMed
-
- Furuichi K, Rivera J, Isersky C. The fate of IgE bound to rat basophilic leukemia cells. III. Relationship between antigen-induced endocytosis and serotonin release. J Immunol. 1984;133:1513–1520. - PubMed
-
- MacGlashan D W, Jr, Mogowski M, Lichtenstein L M. Studies of antigen binding on human basophils. II. Continued expression of antigen-specific IgE during antigen-induced desensitization. J Immunol. 1983;130:2337–2342. - PubMed
-
- MacGlashan D W., Jr Endocytosis, re-cycling and degradation of unoccupied FcεRI in human basophils. J Leukoc Biol. 2007;82:1003–1010. - PubMed
-
- Pierini L, Harris N T, Holowka D, Baird B. Evidence supporting a role for microfilaments in regulating the coupling between poorly dissociable IgE-Fc εRI aggregates downstream signaling pathways. Biochemistry. 1997;36:7447–7456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

