An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model
- PMID: 19150864
- PMCID: PMC2647586
- DOI: 10.1161/STROKEAHA.108.533760
An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model
Abstract
Background and purpose: The potential for successful treatment of intracranial aneurysms by flow diversion is gradually being recognized in the clinical setting; however, the devices currently available (stents) are not designed for flow diversion. We evaluate the long-term response of an appropriately designed flow diversion device in producing thrombotic occlusion of experimental aneurysms.
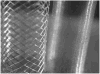
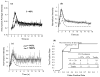
Methods: Three different configurations of an original flow diversion device were implanted across thirty elastase-induced aneurysm models in rabbits. Ten animals per device configuration were followed-up for 3 weeks (n=3), 3 months (n=3), or 6 months (n=4), and tissue explanted postsacrifice was sent for histology. The temporal variation in angiographic contrast intensity within each aneurysm was fitted with a mathematical model to quantify the alteration in local hemodynamics caused by the implanted device. A predictive index, called the washout coefficient, was constructed to estimate long-term aneurysm occlusion probabilities immediately after treatment with any flow diversion device.
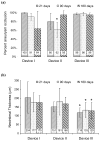
Results: The device with a porosity of 70% and pore density of 18 pores/mm(2) performed better at occluding aneurysms than devices with 70% porosity, 12 pores/mm(2) and 65% porosity, 14 pores/mm(2). A value of the washout coefficient less than 30 predicted greater than 97% angiographic aneurysm occlusion over a period of 6 months with a sensitivity of 73% and specificity of 82%.
Conclusions: The flow diversion devices effected successful and stable aneurysm occlusion. Pore density, rather than porosity, may be the critical factor modulating efficacy of such devices.
Figures

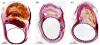

Similar articles
-
Treatment of rabbit elastase-induced aneurysm models by flow diverters: development of quantifiable indexes of device performance using digital subtraction angiography.IEEE Trans Med Imaging. 2009 Jul;28(7):1117-25. doi: 10.1109/TMI.2008.2012162. Epub 2009 Jan 19. IEEE Trans Med Imaging. 2009. PMID: 19164085 Free PMC article.
-
Preclinical Testing of a Novel Thin Film Nitinol Flow-Diversion Stent in a Rabbit Elastase Aneurysm Model.AJNR Am J Neuroradiol. 2016 Mar;37(3):497-501. doi: 10.3174/ajnr.A4568. Epub 2015 Oct 22. AJNR Am J Neuroradiol. 2016. PMID: 26494695 Free PMC article.
-
Evaluation of a newly designed flow diverter for the treatment of intracranial aneurysms in an elastase-induced aneurysm model, in New Zealand white rabbits.Neuroradiology. 2014 Feb;56(2):129-37. doi: 10.1007/s00234-014-1320-8. Neuroradiology. 2014. PMID: 24496551
-
From bench to bedside: utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment.J Neurointerv Surg. 2016 May;8(5):521-5. doi: 10.1136/neurintsurg-2015-011704. Epub 2015 Apr 22. J Neurointerv Surg. 2016. PMID: 25904642 Free PMC article. Review.
-
Flow Diversion Technologies in Evolution: A Review of the First Two Generations of Flow Diversion Devices.World Neurosurg. 2015 Aug;84(2):254-6. doi: 10.1016/j.wneu.2015.03.010. Epub 2015 Mar 28. World Neurosurg. 2015. PMID: 25827045 Review.
Cited by
-
Endovascular treatment with flow diverters may fail to occlude experimental bifurcation aneurysms.Neuroradiology. 2013 Nov;55(11):1355-63. doi: 10.1007/s00234-013-1272-4. Epub 2013 Aug 29. Neuroradiology. 2013. PMID: 23989462
-
The Evolution of Flow-Diverting Stents for Cerebral Aneurysms; Historical Review, Modern Application, Complications, and Future Direction.J Korean Neurosurg Soc. 2020 Mar;63(2):137-152. doi: 10.3340/jkns.2020.0034. Epub 2020 Feb 27. J Korean Neurosurg Soc. 2020. PMID: 32120455 Free PMC article.
-
A review of technological innovations leading to modern endovascular brain aneurysm treatment.Front Neurol. 2023 Apr 11;14:1156887. doi: 10.3389/fneur.2023.1156887. eCollection 2023. Front Neurol. 2023. PMID: 37114225 Free PMC article. Review.
-
A new-generation, low-permeability flow diverting device for treatment of saccular aneurysms.Eur Radiol. 2014 Jan;24(1):12-8. doi: 10.1007/s00330-013-2970-3. Epub 2013 Jul 24. Eur Radiol. 2014. PMID: 23881302
-
Analysis and quantification of endovascular coil distribution inside saccular aneurysms using histological images.J Neurointerv Surg. 2013 Nov;5 Suppl 3(0 3):iii33-7. doi: 10.1136/neurintsurg-2012-010456. Epub 2012 Aug 21. J Neurointerv Surg. 2013. PMID: 22914746 Free PMC article.
References
-
- Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–4. - PubMed
-
- Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17. - PubMed
-
- Pelz DM, Levy EI, Hopkins LN. Advances in interventional neuroradiology 2007. Stroke. 2008;39:268–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

