The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging
- PMID: 19150923
- PMCID: PMC2742588
- DOI: 10.1093/cercor/bhn242
The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging
Abstract
Whereas neuronal numbers are largely preserved in normal aging, subtle morphological changes occur in dendrites and spines, whose electrotonic consequences remain unexplored. We examined age-related morphological alterations in 2 types of pyramidal neurons contributing to working memory circuits in the macaque prefrontal cortex (PFC): neurons in the superior temporal cortex forming "long" projections to the PFC and "local" projection neurons within the PFC. Global dendritic mass homeostasis, measured by 3-dimensional scaling analysis, was conserved with aging in both neuron types. Spine densities, dendrite diameters, lengths, and branching complexity were all significantly reduced in apical dendrites of long projection neurons with aging, but only spine parameters were altered in local projection neurons. Despite these differences, voltage attenuation due to passive electrotonic structure, assuming equivalent cable parameters, was significantly reduced with aging in the apical dendrites of both neuron classes. Confirming the electrotonic analysis, simulated passive backpropagating action potential efficacy was significantly higher in apical but not basal dendrites of old neurons. Unless compensated by changes in passive cable parameters, active membrane properties, or altered synaptic properties, these effects will increase the excitability of pyramidal neurons, compromising the precisely tuned activity required for working memory, ultimately resulting in age-related PFC dysfunction.
Figures
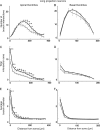



 in) and outward (
in) and outward ( out) attenuation transforms at DC in long projection neurons. Histograms show means ± standard error of the mean (error bars) of
out) attenuation transforms at DC in long projection neurons. Histograms show means ± standard error of the mean (error bars) of  out (A, B) and
out (A, B) and  in (C, D) for the young (black bars) and old (gray bars) neuron groups. In each cell (A–D), mean attenuation transforms were compared for models without spines (left panels) or including spines (right panels). Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01). Values represent means ± standard error of the mean.
in (C, D) for the young (black bars) and old (gray bars) neuron groups. In each cell (A–D), mean attenuation transforms were compared for models without spines (left panels) or including spines (right panels). Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01). Values represent means ± standard error of the mean.
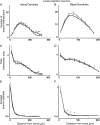
 in) and outward (
in) and outward ( out) attenuation transforms at DC in local projection neurons. Format as in Figure 6.
out) attenuation transforms at DC in local projection neurons. Format as in Figure 6.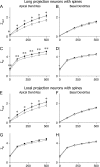
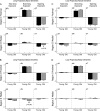
 in and
in and  out transforms in young (solid black line) and old (dashed lines) neurons. Plotted points at each 100 Hz interval represent means ± standard error of the mean. Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01). Data series in (H) were virtually identical: aged neuron data have been shifted downward by a value of 0.1 on the log attenuation axis to aid visualization.
out transforms in young (solid black line) and old (dashed lines) neurons. Plotted points at each 100 Hz interval represent means ± standard error of the mean. Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01). Data series in (H) were virtually identical: aged neuron data have been shifted downward by a value of 0.1 on the log attenuation axis to aid visualization.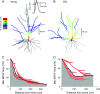
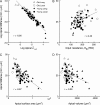
 out is the best predictor of NBParea, with a strong linear negative correlation that becomes even stronger on a log-log scale. (B) Input resistance at the base of the apical trunk, RN, showed a moderate, positive correlation; (C) total surface area showed a moderate, negative correlation; and (D) total volume was poorly correlated with backpropagation efficacy.
out is the best predictor of NBParea, with a strong linear negative correlation that becomes even stronger on a log-log scale. (B) Input resistance at the base of the apical trunk, RN, showed a moderate, positive correlation; (C) total surface area showed a moderate, negative correlation; and (D) total volume was poorly correlated with backpropagation efficacy.Similar articles
-
Quantitative analysis of the dendritic morphology of corticocortical projection neurons in the macaque monkey association cortex.Neuroscience. 2002;114(2):349-59. doi: 10.1016/s0306-4522(02)00305-6. Neuroscience. 2002. PMID: 12204204
-
Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys.Cereb Cortex. 2003 Sep;13(9):950-61. doi: 10.1093/cercor/13.9.950. Cereb Cortex. 2003. PMID: 12902394
-
Functionally relevant measures of spatial complexity in neuronal dendritic arbors.J Theor Biol. 2006 Feb 7;238(3):505-26. doi: 10.1016/j.jtbi.2005.06.001. Epub 2005 Aug 3. J Theor Biol. 2006. PMID: 16083911
-
Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex.Neuropsychopharmacology. 1999 Aug;21(2):161-94. doi: 10.1016/S0893-133X(98)00112-2. Neuropsychopharmacology. 1999. PMID: 10432466 Review.
-
Effects of normal aging on prefrontal area 46 in the rhesus monkey.Brain Res Rev. 2010 Mar;62(2):212-32. doi: 10.1016/j.brainresrev.2009.12.002. Epub 2009 Dec 11. Brain Res Rev. 2010. PMID: 20005254 Free PMC article. Review.
Cited by
-
Functional consequences of age-related morphologic changes to pyramidal neurons of the rhesus monkey prefrontal cortex.J Comput Neurosci. 2015 Apr;38(2):263-83. doi: 10.1007/s10827-014-0541-5. Epub 2014 Dec 20. J Comput Neurosci. 2015. PMID: 25527184 Free PMC article.
-
How do neurons age? A focused review on the aging of the microtubular cytoskeleton.Neural Regen Res. 2024 Sep 1;19(9):1899-1907. doi: 10.4103/1673-5374.390974. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227514 Free PMC article.
-
Selective Loss of Thin Spines in Area 7a of the Primate Intraparietal Sulcus Predicts Age-Related Working Memory Impairment.J Neurosci. 2018 Dec 5;38(49):10467-10478. doi: 10.1523/JNEUROSCI.1234-18.2018. Epub 2018 Oct 24. J Neurosci. 2018. PMID: 30355632 Free PMC article.
-
Lipid droplets in the nervous system.J Cell Biol. 2021 Jul 5;220(7):e202102136. doi: 10.1083/jcb.202102136. Epub 2021 Jun 21. J Cell Biol. 2021. PMID: 34152362 Free PMC article. Review.
-
A general homeostatic principle following lesion induced dendritic remodeling.Acta Neuropathol Commun. 2016 Feb 25;4:19. doi: 10.1186/s40478-016-0285-8. Acta Neuropathol Commun. 2016. PMID: 26916562 Free PMC article.
References
-
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12:99–111. - PubMed
-
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol. 1986;252:415–422. - PubMed
-
- Brown TH, Zador A, Mainen ZF, Claiborne BJ. Hebbian computations in hippocampal dendrites and spines. In: McKenna T, Davis J, Zornetzer SF, editors. Single neuron computation. San Diego (CA): Academic Press; 1992. pp. 81–116.
-
- Bui TV, Cushing S, Dewey D, Fyffe RE, Rose PK. Comparison of the morphological and electrotonic properties of Renshaw cells, Ia inhibitory interneurons, and motoneurons in the cat. J Neurophysiol. 2003;90:2900–2918. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

