Identification and functional analysis of a novel cyclin e/cdk2 substrate ankrd17
- PMID: 19150984
- PMCID: PMC2658080
- DOI: 10.1074/jbc.M807827200
Identification and functional analysis of a novel cyclin e/cdk2 substrate ankrd17
Abstract
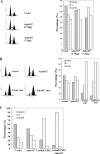
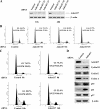
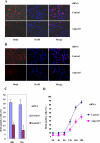
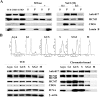
Cyclin E/Cdk2 is a key regulator in G(1)-S transition. We have identified a novel cyclin E/Cdk2 substrate called Ankrd17 (ankyrin repeat protein 17) using the TAP tag purification technique. Ankrd17 protein contains two clusters of a total 25 ankyrin repeats at its N terminus, one NES (nuclear exporting signal) and one NLS (nuclear localization signal) in the middle, and one RXL motif at its C terminus. Ankrd17 is expressed in various tissues and associates with cyclin E/Cdk2 in an RXL-dependent manner. It can be phosphorylated by cyclin E/Cdk2 at 3 phosphorylation sites (Ser(1791), Ser(1794), and Ser(2150)). Overexpression of Ankrd17 promotes S phase entry, whereas depletion of Ankrd17 expression by small interfering RNA inhibits DNA replication and blocks cell cycle progression as well as up-regulates the expression of p53 and p21. Ankrd17 is localized to the nucleus and interacts with DNA replication factors including MCM family members, Cdc6 and PCNA. Depletion of Ankrd17 results in decreased loading of Cdc6 and PCNA onto DNA suggesting that Ankrd17 may be directly involved in the DNA replication process. Taken together, these data indicate that Ankrd17 is an important downstream effector of cyclin E/Cdk2 and positively regulates G(1)/S transition.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

