Identifying and characterizing a functional HIV-1 reverse transcriptase-binding site on integrase
- PMID: 19150986
- PMCID: PMC2658086
- DOI: 10.1074/jbc.M806241200
Identifying and characterizing a functional HIV-1 reverse transcriptase-binding site on integrase
Abstract
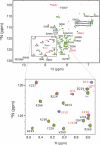
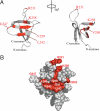
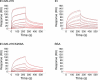
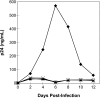
Integrase (IN) from human immunodeficiency virus, type 1 (HIV-1) exerts pleiotropic effects in the viral replication cycle. Besides integration, IN mutations can impact nuclear import, viral maturation, and reverse transcription. IN and reverse transcriptase (RT) interact in vitro, and the IN C-terminal domain (CTD) is both necessary and sufficient for binding RT. We used nuclear magnetic resonance spectroscopy to identify a putative RT-binding surface on the IN CTD, and surface plasmon resonance to obtain kinetic parameters and the binding affinity for the IN-RT interaction. An IN K258A substitution that disrupts reverse transcription in infected cells is located at the putative RT-binding surface, and we found that this substitution substantially weakens IN CTD-RT interactions. We also identified two additional IN amino acid substitutions located at the putative RT-binding surface (W243E and V250E) that significantly impair viral replication in tissue culture. These results strengthen the notion that IN-RT interactions are biologically relevant during HIV-1 replication and also provide insights into this interaction at the molecular level.
Figures

Similar articles
-
Interaction between Reverse Transcriptase and Integrase Is Required for Reverse Transcription during HIV-1 Replication.J Virol. 2015 Dec;89(23):12058-69. doi: 10.1128/JVI.01471-15. Epub 2015 Sep 23. J Virol. 2015. PMID: 26401032 Free PMC article.
-
Identification of residues in the C-terminal domain of HIV-1 integrase that mediate binding to the transportin-SR2 protein.J Biol Chem. 2012 Oct 5;287(41):34059-68. doi: 10.1074/jbc.M112.387944. Epub 2012 Aug 7. J Biol Chem. 2012. PMID: 22872638 Free PMC article.
-
Critical Contribution of Tyr15 in the HIV-1 Integrase (IN) in Facilitating IN Assembly and Nonenzymatic Function through the IN Precursor Form with Reverse Transcriptase.J Virol. 2016 Dec 16;91(1):e02003-16. doi: 10.1128/JVI.02003-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795445 Free PMC article.
-
The structural biology of HIV-1: mechanistic and therapeutic insights.Nat Rev Microbiol. 2012 Mar 16;10(4):279-90. doi: 10.1038/nrmicro2747. Nat Rev Microbiol. 2012. PMID: 22421880 Free PMC article. Review.
-
The C-Terminal Domain of HIV-1 Integrase: A Swiss Army Knife for the Virus?Viruses. 2022 Jun 27;14(7):1397. doi: 10.3390/v14071397. Viruses. 2022. PMID: 35891378 Free PMC article. Review.
Cited by
-
The removal of RNA primers from DNA synthesized by the reverse transcriptase of the retrotransposon Tf1 is stimulated by Tf1 integrase.J Virol. 2012 Jun;86(11):6222-30. doi: 10.1128/JVI.00009-12. Epub 2012 Apr 4. J Virol. 2012. PMID: 22491446 Free PMC article.
-
Histone deacetylase 1 interacts with HIV-1 Integrase and modulates viral replication.Virol J. 2019 Nov 19;16(1):138. doi: 10.1186/s12985-019-1249-y. Virol J. 2019. PMID: 31744547 Free PMC article.
-
Inhibiting the HIV integration process: past, present, and the future.J Med Chem. 2014 Feb 13;57(3):539-66. doi: 10.1021/jm400674a. Epub 2013 Sep 25. J Med Chem. 2014. PMID: 24025027 Free PMC article.
-
HIV-1 B-subtype capsid protein: a characterization of amino acid's conservation and its significant association with integrase signatures.Virus Genes. 2014 Jun;48(3):429-37. doi: 10.1007/s11262-014-1039-y. Epub 2014 Jan 29. Virus Genes. 2014. PMID: 24474329
-
Altered viral fitness and drug susceptibility in HIV-1 carrying mutations that confer resistance to nonnucleoside reverse transcriptase and integrase strand transfer inhibitors.J Virol. 2014 Aug;88(16):9268-76. doi: 10.1128/JVI.00695-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899199 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

