Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals
- PMID: 19151191
- PMCID: PMC2858810
- DOI: 10.1164/rccm.200809-1486OC
Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals
Abstract
Rationale: An effective new tuberculosis (TB) vaccine regimen must be safe in individuals with latent TB infection (LTBI) and is a priority for global health care.
Objectives: To evaluate the safety and immunogenicity of a leading new TB vaccine, recombinant Modified Vaccinia Ankara expressing Antigen 85A (MVA85A) in individuals with LTBI.
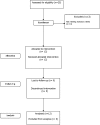
Methods: An open-label, phase I trial of MVA85A was performed in 12 subjects with LTBI recruited from TB contact clinics in Oxford and London or by poster advertisements in Oxford hospitals. Patients were assessed clinically and had blood samples drawn for immunological analysis over a 52-week period after vaccination with MVA85A. Thoracic computed tomography scans were performed at baseline and at 10 weeks after vaccination. Safety of MVA85A was assessed by clinical, radiological, and inflammatory markers. The immunogenicity of MVA85A was assessed by IFNgamma and IL-2 ELISpot assays and FACS.
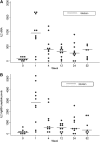
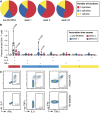
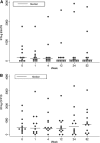
Measurements and main results: MVA85A was safe in subjects with LTBI, with comparable adverse events to previous trials of MVA85A. There were no clinically significant changes in inflammatory markers or thoracic computed tomography scans after vaccination. MVA85A induced a strong antigen-specific IFN-gamma and IL-2 response that was durable for 52 weeks. The magnitude of IFN-gamma response was comparable to previous trials of MVA85A in bacillus Calmette-Guérin-vaccinated individuals. Antigen 85A-specific polyfunctional CD4(+) T cells were detectable prior to vaccination with statistically significant increases in cell numbers after vaccination.
Conclusions: MVA85A is safe and highly immunogenic in individuals with LTBI. These results will facilitate further trials in TB-endemic areas. Clinical trial registered with www.clinicaltrials.gov (NCT00456183).
Figures


Comment in
-
A courageous step down the road toward a new tuberculosis vaccine.Am J Respir Crit Care Med. 2009 Apr 15;179(8):628-9. doi: 10.1164/rccm.200901-0144ED. Am J Respir Crit Care Med. 2009. PMID: 19351870 No abstract available.
Similar articles
-
Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial.PLoS Med. 2019 Apr 30;16(4):e1002790. doi: 10.1371/journal.pmed.1002790. eCollection 2019 Apr. PLoS Med. 2019. PMID: 31039172 Free PMC article. Clinical Trial.
-
Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial.Lancet Respir Med. 2015 Mar;3(3):190-200. doi: 10.1016/S2213-2600(15)00037-5. Epub 2015 Feb 26. Lancet Respir Med. 2015. PMID: 25726088 Free PMC article. Clinical Trial.
-
Serum indoleamine 2,3-dioxygenase activity is associated with reduced immunogenicity following vaccination with MVA85A.BMC Infect Dis. 2014 Dec 3;14:660. doi: 10.1186/s12879-014-0660-7. BMC Infect Dis. 2014. PMID: 25466778 Free PMC article. Clinical Trial.
-
The Systematic Review and Meta-Analysis on the Immunogenicity and Safety of the Tuberculosis Subunit Vaccines M72/AS01E and MVA85A.Front Immunol. 2020 Oct 8;11:1806. doi: 10.3389/fimmu.2020.01806. eCollection 2020. Front Immunol. 2020. PMID: 33133057 Free PMC article.
-
Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis.Vaccine. 2014 Feb 3;32(6):712-6. doi: 10.1016/j.vaccine.2013.11.065. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24300592 Review.
Cited by
-
Goats primed with Mycobacterium bovis BCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis.Clin Vaccine Immunol. 2012 Sep;19(9):1339-47. doi: 10.1128/CVI.00275-12. Epub 2012 Jul 3. Clin Vaccine Immunol. 2012. PMID: 22761299 Free PMC article.
-
Identification of major factors influencing ELISpot-based monitoring of cellular responses to antigens from Mycobacterium tuberculosis.PLoS One. 2009 Nov 24;4(11):e7972. doi: 10.1371/journal.pone.0007972. PLoS One. 2009. PMID: 19956718 Free PMC article.
-
Novel GMO-Based Vaccines against Tuberculosis: State of the Art and Biosafety Considerations.Vaccines (Basel). 2014 Jun 16;2(2):463-99. doi: 10.3390/vaccines2020463. Vaccines (Basel). 2014. PMID: 26344627 Free PMC article. Review.
-
Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery.Vaccine. 2013 Feb 4;31(7):1026-33. doi: 10.1016/j.vaccine.2012.12.042. Epub 2012 Dec 21. Vaccine. 2013. PMID: 23266342 Free PMC article. Clinical Trial.
-
Development of an antibody to bovine IL-2 reveals multifunctional CD4 T(EM) cells in cattle naturally infected with bovine tuberculosis.PLoS One. 2011;6(12):e29194. doi: 10.1371/journal.pone.0029194. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216206 Free PMC article.
References
-
- Global tuberculosis control: surveillance, planning, financing. WHO Report 2007 [Internet]. Geneva, Switzerland: World Health Organization; 2007. (WHO/HTM/TB/2007.376). (Accessed 2009 Feb 11). Available from: http://www.who.int/tb/publications/global_report/2007/contents/en/index....
-
- Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol 2001;55:139–163. - PubMed
-
- Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, Gey van Pittius NC, van Helden PD. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 2004;169:610–614. - PubMed
-
- Cappelli G, Volpe P, Sanduzzi A, Sacchi A, Colizzi V, Mariani F. Human macrophage gamma interferon decreases gene expression but not replication of Mycobacterium tuberculosis: analysis of the host-pathogen reciprocal influence on transcription in a comparison of strains H37Rv and CMT97. Infect Immun 2001;69:7262–7270. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

