Postnatal estradiol up-regulates lung nitric oxide synthases and improves lung function in bronchopulmonary dysplasia
- PMID: 19151197
- PMCID: PMC2654978
- DOI: 10.1164/rccm.200805-794OC
Postnatal estradiol up-regulates lung nitric oxide synthases and improves lung function in bronchopulmonary dysplasia
Abstract
Rationale: Nitric oxide (NO) plays an important role in lung development and perinatal lung function, and pulmonary NO synthases (NOS) are decreased in bronchopulmonary dysplasia (BPD) following preterm birth. Fetal estradiol levels increase during late gestation and estradiol up-regulates NOS, suggesting that after preterm birth estradiol deprivation causes attenuated lung NOS resulting in impaired pulmonary function.
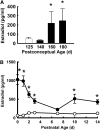
Objective: To test the effects of postnatal estradiol administration in a primate model of BPD over 14 days after delivery at 125 days of gestation (term = 185 d).
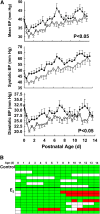
Methods: Cardiopulmonary function was assessed by echocardiography and whole body plethysmography. Lung morphometric and histopathologic analyses were performed, and NOS enzymatic activity and abundance were measured.
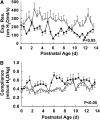
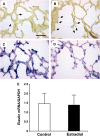
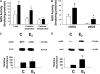
Measurements and main results: Estradiol caused an increase in blood pressure and ductus arteriosus closure. Expiratory resistance and lung compliance were also improved, and this occurred before spontaneous ductal closure. Furthermore, both oxygenation and ventilation indices were improved with estradiol, and the changes in lung function and ventilatory support requirements persisted throughout the study period. Whereas estradiol had negligible effect on indicators of lung inflammation and on lung structure assessed after the initial 14 days of ventilatory support, it caused an increase in lung neuronal and endothelial NOS enzymatic activity.
Conclusions: In a primate model of BPD, postnatal estradiol treatment had favorable cardiovascular impact, enhanced pulmonary function, and lowered requirements for ventilatory support in association with an up-regulation of lung NOS. Estradiol may be an efficacious postnatal therapy to improve lung function and outcome in preterm infants.
Figures


References
-
- Barnes PJ. Nitric oxide and airway disease. Ann Med 1995;27:389–393. - PubMed
-
- Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. - PubMed
-
- Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase (eNOS) deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. - PubMed
-
- Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol 2002;282:L379–L385. - PubMed
-
- Tang ZL, Wasserloos KJ, Liu X, Stitt MS, Reynolds IJ, Pitt BR, St Croix CM. Nitric oxide decreases the sensitivity of pulmonary endothelial cells to LPS-induced apoptosis in a zinc-dependent fashion. Mol Cell Biochem 2002;234–235:211–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

