Revisiting the cellular mechanisms of strong luminal alkalinization in the anterior midgut of larval mosquitoes
- PMID: 19151212
- PMCID: PMC2706088
- DOI: 10.1242/jeb.023580
Revisiting the cellular mechanisms of strong luminal alkalinization in the anterior midgut of larval mosquitoes
Abstract
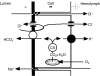
Here we critically review two recent hypotheses about the mechanism of strong alkalinization by the anterior midgut of mosquito larvae and our tests of these hypotheses. We present experimental evidence against the major components of transport models proposed in these hypotheses. Measurements of the transapical and transbasal proton electrochemical gradients provide an indication of driving forces faced by and generated by the transport mechanisms of the tissue. These measurements confirmed that basal V-ATPase energizes alkalinization. Serotonin stimulates the V-ATPase, as indicated by the ensuing increase in proton-motive force across the basal membrane. Moreover, the neurohormone resulted in a surprisingly large increase in the intracellular pH. The results of inhibitor studies indicate that, contrary to previous proposals, carbonic anhydrase is apparently not involved in supplying acid-base equivalents to the respective transporters. Furthermore, any apical processes proposed to be involved in alkali secretion or acid absorption must be Cl(-) independent and insensitive to DIDS, amiloride, Zn(2+) and ouabain. These results argue against the involvement of putative apical Cl(-)/HCO (-)(3) exchangers, apical H(+) channels, apical cation/proton exchangers and the importance of the apical Na(+)/K(+) pump. The studies analyzed here thus provide both a limitation and direction for further studies of the mechanism of strong alkalinization in this system.
Figures



Similar articles
-
Cationic pathway of pH regulation in larvae of Anopheles gambiae.J Exp Biol. 2008 Mar;211(Pt 6):957-68. doi: 10.1242/jeb.012021. J Exp Biol. 2008. PMID: 18310121
-
Evidence for HCO3- and NH3/NH4+-dependent pH regulatory mechanisms in the alkaline midgut of the sea urchin larva.Am J Physiol Regul Integr Comp Physiol. 2025 Jun 1;328(6):R685-R699. doi: 10.1152/ajpregu.00222.2024. Epub 2025 Apr 18. Am J Physiol Regul Integr Comp Physiol. 2025. PMID: 40248920
-
Alkalinization by chloride/bicarbonate pathway in larval mosquito midgut.Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15354-9. doi: 10.1073/pnas.261253998. Epub 2001 Dec 11. Proc Natl Acad Sci U S A. 2001. PMID: 11742083 Free PMC article.
-
The key role of the H+ V-ATPase in acid-base balance and Na+ transport processes in frog skin.J Exp Biol. 1997 Jan;200(Pt 2):247-56. doi: 10.1242/jeb.200.2.247. J Exp Biol. 1997. PMID: 9050232 Review.
-
The insect V-ATPase, a plasma membrane proton pump energizing secondary active transport: molecular analysis of electrogenic potassium transport in the tobacco hornworm midgut.J Exp Biol. 1992 Nov;172:335-43. doi: 10.1242/jeb.172.1.335. J Exp Biol. 1992. PMID: 1491230 Review.
Cited by
-
Na+/K+-ATPase Activation by cAMP in the Midgut of Lutzomyia longipalpis (Lutz & Neiva, 1912; Diptera: Psychodidae).J Insect Sci. 2022 Mar 1;22(2):1. doi: 10.1093/jisesa/ieac008. J Insect Sci. 2022. PMID: 35271719 Free PMC article.
-
Carbonic anhydrases and anion transport in mosquito midgut pH regulation.J Exp Biol. 2009 Jun;212(Pt 11):1662-71. doi: 10.1242/jeb.028084. J Exp Biol. 2009. PMID: 19448076 Free PMC article. Review.
-
Ion and solute transport by Prestin in Drosophila and Anopheles.J Insect Physiol. 2012 Apr;58(4):563-9. doi: 10.1016/j.jinsphys.2012.01.009. Epub 2012 Jan 30. J Insect Physiol. 2012. PMID: 22321763 Free PMC article.
-
Fluid absorption in the isolated midgut of adult female yellow fever mosquitoes (Aedes aegypti).J Exp Biol. 2015 Jul;218(Pt 13):2023-9. doi: 10.1242/jeb.119529. Epub 2015 May 5. J Exp Biol. 2015. PMID: 25944920 Free PMC article.
-
Voltage coupling of primary H+ V-ATPases to secondary Na+- or K+-dependent transporters.J Exp Biol. 2009 Jun;212(Pt 11):1620-9. doi: 10.1242/jeb.031534. J Exp Biol. 2009. PMID: 19448072 Free PMC article. Review.
References
-
- Azuma, M., Harvey, W. R. and Wieczorek, H. (1995). Stoichiometry of K+/H+ antiport helps to explain extracellular pH 11 in a model epithelium. FEBS Lett. 361, 153-156. - PubMed
-
- Beyenbach, K. W. (2001). Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol. Sci. 16, 145-151. - PubMed
-
- Clark, T. M., Koch, A. and Moffett, D. F. (1999). The anterior and posterior `stomach' regions of larval Aedes aegypti midgut: regional specialization of ion transport and stimulation by 5-hydroxytryptamine. J. Exp. Biol. 202, 247-252. - PubMed
-
- Clark, T. M., Koch, A. and Moffett, D. F. (2000). The electrical properties of the anterior stomach of the larval mosquito (Aedes aegypti). J. Exp. Biol. 203, 1093-1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

