Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease
- PMID: 19151254
- PMCID: PMC12616545
- DOI: 10.1152/ajpheart.00900.2008
Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease
Abstract
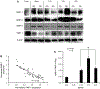
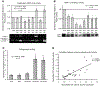
Matrix metalloproteinases (MMPs) and cathepsins are proteolytic enzymes that are upregulated in diseased aortic valve cusps. The objective of this study was to investigate whether elevated cyclic stretch causes an increased expression and activity of these proteolytic enzymes in the valve cusp. Circumferentially oriented fresh porcine aortic valve cusp sections were stretched to 10% (physiological), 15% (pathological), and 20% (hyperpathological) in a tensile stretch bioreactor for 24 and 48 h. The expression and activity of MMP-1, MMP-2, MMP-9, tissue inhibitor of MMP-1, and cathepsin L, S, and K were quantified and compared with fresh controls. Cell proliferation and apoptosis were also analyzed. As a result, at 10% physiological stretch, the expression and activity of remodeling enzymes were comparable with fresh controls. At 15% stretch, the expression of MMP-1, -2, -9 and cathepsin S and K were upregulated, whereas the expression of cathepsin L was downregulated compared with controls. A similar trend was observed at 20% stretch, but the magnitudes of upregulation and downregulation of the expression were less than those observed at 15%. In addition, there were significantly higher cell proliferation and apoptosis at 20% stretch compared with those of other treatment groups. In conclusion, elevated mechanical stretch on aortic valve cusps may detrimentally alter the proteolytic enzyme expression and activity in valve cells. This may trigger a cascade of events leading to an accelerated valve degeneration and disease progression.
Figures



References
-
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin 24: 135–146, 2006. - PubMed
-
- Billiar KL, Sacks MS. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II—A structural constitutive model. J Biomech Eng 122: 327–335, 2000. - PubMed
-
- Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp—Part I: Experimental results. J Biomech Eng 122: 23–30, 2000. - PubMed
-
- Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol 200: 423–428, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

