Sulfonylurea receptor 1 mutations that cause opposite insulin secretion defects with chemical chaperone exposure
- PMID: 19151370
- PMCID: PMC2658088
- DOI: 10.1074/jbc.M807012200
Sulfonylurea receptor 1 mutations that cause opposite insulin secretion defects with chemical chaperone exposure
Abstract
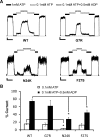
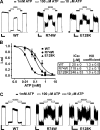
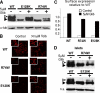
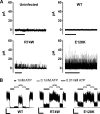
The beta-cell ATP-sensitive potassium (K(ATP)) channel composed of sulfonylurea receptor SUR1 and potassium channel Kir6.2 serves a key role in insulin secretion regulation by linking glucose metabolism to cell excitability. Mutations in SUR1 or Kir6.2 that decrease channel function are typically associated with congenital hyperinsulinism, whereas those that increase channel function are associated with neonatal diabetes. Here we report that two hyperinsulinism-associated SUR1 missense mutations, R74W and E128K, surprisingly reduce channel inhibition by intracellular ATP, a gating defect expected to yield the opposite disease phenotype neonatal diabetes. Under normal conditions, both mutant channels showed poor surface expression due to retention in the endoplasmic reticulum, accounting for the loss of channel function phenotype in the congenital hyperinsulinism patients. This trafficking defect, however, could be corrected by treating cells with the oral hypoglycemic drugs sulfonylureas, which we have shown previously to act as small molecule chemical chaperones for K(ATP) channels. The R74W and E128K mutants thus rescued to the cell surface paradoxically exhibited ATP sensitivity 6- and 12-fold lower than wild-type channels, respectively. Further analyses revealed a nucleotide-independent decrease in mutant channel intrinsic open probability, suggesting the mutations may reduce ATP sensitivity by causing functional uncoupling between SUR1 and Kir6.2. In insulin-secreting cells, rescue of both mutant channels to the cell surface led to hyperpolarized membrane potentials and reduced insulin secretion upon glucose stimulation. Our results show that sulfonylureas, as chemical chaperones, can dictate manifestation of the two opposite insulin secretion defects by altering the expression levels of the disease mutants.
Figures



Similar articles
-
Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism.J Biol Chem. 2008 Apr 4;283(14):9146-56. doi: 10.1074/jbc.M708798200. Epub 2008 Feb 4. J Biol Chem. 2008. PMID: 18250167 Free PMC article.
-
N-terminal transmembrane domain of SUR1 controls gating of Kir6.2 by modulating channel sensitivity to PIP2.J Gen Physiol. 2011 Mar;137(3):299-314. doi: 10.1085/jgp.201010557. Epub 2011 Feb 14. J Gen Physiol. 2011. PMID: 21321069 Free PMC article.
-
Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue.Diabetes. 2007 Sep;56(9):2339-48. doi: 10.2337/db07-0150. Epub 2007 Jun 15. Diabetes. 2007. PMID: 17575084 Free PMC article.
-
Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism.Hum Mutat. 2009 Feb;30(2):170-80. doi: 10.1002/humu.20838. Hum Mutat. 2009. PMID: 18767144 Review.
-
Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism.Hum Mutat. 2006 Mar;27(3):220-31. doi: 10.1002/humu.20292. Hum Mutat. 2006. PMID: 16416420 Review.
Cited by
-
KATP channel mutations in congenital hyperinsulinism: Progress and challenges towards mechanism-based therapies.Front Endocrinol (Lausanne). 2023 Mar 28;14:1161117. doi: 10.3389/fendo.2023.1161117. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37056678 Free PMC article. Review.
-
Engineered interaction between SUR1 and Kir6.2 that enhances ATP sensitivity in KATP channels.J Gen Physiol. 2012 Aug;140(2):175-87. doi: 10.1085/jgp.201210803. Epub 2012 Jul 16. J Gen Physiol. 2012. PMID: 22802363 Free PMC article.
-
Sodium 4-phenylbutyrate ameliorates the effects of cataract-causing mutant gammaD-crystallin in cultured cells.Mol Vis. 2010 Jun 4;16:997-1003. Mol Vis. 2010. PMID: 20577655 Free PMC article.
-
Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM.Elife. 2017 Oct 24;6:e31054. doi: 10.7554/eLife.31054. Elife. 2017. PMID: 29035201 Free PMC article.
-
Small molecule SWELL1 complex induction improves glycemic control and nonalcoholic fatty liver disease in murine Type 2 diabetes.Nat Commun. 2022 Feb 10;13(1):784. doi: 10.1038/s41467-022-28435-0. Nat Commun. 2022. PMID: 35145074 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

