Effectiveness of placebo therapy for maintaining masking in a clinical trial of vergence/accommodative therapy
- PMID: 19151384
- PMCID: PMC2759605
- DOI: 10.1167/iovs.08-2693
Effectiveness of placebo therapy for maintaining masking in a clinical trial of vergence/accommodative therapy
Abstract
Purpose: To evaluate the effectiveness of the Convergence Insufficiency Treatment Trial (CITT) placebo therapy program in maintaining masking of patients randomized to the office-based treatment arms, determine whether demographic variables affect masking, and determine whether perception of assigned treatment group was associated with treatment outcome or adherence to treatment.
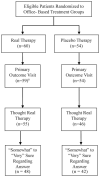
Methods: Patients (n = 221, ages, 9-17 years) were randomized to one of four treatment groups, two of which were office-based and masked to treatment (n = 114). The placebo therapy program was designed to appear to be real vergence/accommodative therapy, without stimulating vergence, accommodation, or fine saccades (beyond levels of daily visual activities). After treatment, patients in the office-based groups were asked whether they thought they had received real or placebo therapy and how confident they were in their answers.
Results: Ninety-three percent of patients assigned to real therapy and 85% assigned to placebo therapy thought they were in the real therapy group (P = 0.17). No significant differences were found between the two groups in adherence to the therapy (P >or= 0.22 for all comparisons). The percentage of patients who thought they were assigned to real therapy did not differ by age, sex, race, or ethnicity (P > 0.30 for all comparisons). No association was found between patients' perception of group assignment and symptoms or signs at outcome (P >or= 0.38 for all comparisons).
Conclusions: The CITT placebo therapy program was effective in maintaining patient masking in this study and therefore may have potential for use in future clinical trials using vergence/accommodative therapy. Masking was not affected by demographic variables. Perception of group assignment was not related to symptoms or signs at outcome (ClinicalTrials.gov number, NCT00338611).
Figures
References
-
- Borsting EJ, Rouse MW, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80:832–838. - PubMed
-
- Rouse MW, Borsting EJ, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic Physiol Opt. 2004;24:384–390. - PubMed
-
- Borsting E, Rouse MW, Deland PN, et al. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74:25–34. - PubMed
-
- Duke-Elder S, Wybar K. Ocular Motility and Strabismus. System of Ophthalmology. Vol. 6. St. Louis: Mosby; 1973.
-
- Rouse MW, Borsting E, Hyman L, et al. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) group. Optom Vis Sci. 1999;76:643–649. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 EY014712/EY/NEI NIH HHS/United States
- U10EY014676/EY/NEI NIH HHS/United States
- U10EY014713/EY/NEI NIH HHS/United States
- U10 EY014709/EY/NEI NIH HHS/United States
- U10EY014716/EY/NEI NIH HHS/United States
- U10EY014715/EY/NEI NIH HHS/United States
- U10 EY014716/EY/NEI NIH HHS/United States
- U10EY014709/EY/NEI NIH HHS/United States
- U10EY014706/EY/NEI NIH HHS/United States
- U10 EY014676/EY/NEI NIH HHS/United States
- U10 EY014706/EY/NEI NIH HHS/United States
- U10 EY014713/EY/NEI NIH HHS/United States
- U10EY014659/EY/NEI NIH HHS/United States
- U10 EY014715/EY/NEI NIH HHS/United States
- U10 EY014710/EY/NEI NIH HHS/United States
- U10EY014710/EY/NEI NIH HHS/United States
- U10EY014712/EY/NEI NIH HHS/United States
- U10 EY014659/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical