Assessment of the efficacy of chelate-assisted phytoextraction of lead by coffeeweed (Sesbania exaltata Raf.)
- PMID: 19151439
- PMCID: PMC3700004
- DOI: 10.3390/ijerph5050428
Assessment of the efficacy of chelate-assisted phytoextraction of lead by coffeeweed (Sesbania exaltata Raf.)
Abstract
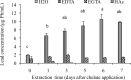
Lead (Pb), depending upon the reactant surface, pH, redox potential and other factors can bind tightly to the soil with a retention time of many centuries. Soil-metal interactions by sorption, precipitation and complexation processes, and differences between plant species in metal uptake efficiency, transport, and susceptibility make a general prediction of soil metal bioavailability and risks of plant metal toxicity difficult. Moreover, the tight binding characteristic of Pb to soils and plant materials make a significant portion of Pb unavailable for uptake by plants. This experiment was conducted to determine whether the addition of ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), or acetic acid (HAc) can enhance the phytoextraction of Pb by making the Pb soluble and more bioavailable for uptake by coffeeweed (Sesbania exaltata Raf.). Also we wanted to assess the efficacy of chelates in facilitating translocation of the metal into the above-ground biomass of this plant. To test the effect of chelates on Pb solubility, 2 g of Pb-spiked soil (1000 mg Pb/kg dry soil) were added to each 15 mL centrifuge tube. Chelates (EDTA, EGTA, HAc) in a 1:1 ratio with the metal, or distilled deionized water were then added. Samples were shaken on a platform shaker then centrifuged at the end of several time periods. Supernatants were filtered with a 0.45 mum filter and quantified by inductively coupled plasma-optical emission spectrometry (ICP-OES) to determine soluble Pb concentrations. Results revealed that EDTA was the most effective in bringing Pb into solution, and that maximum solubility was reached 6 days after chelate amendment. Additionally, a greenhouse experiment was conducted by planting Sesbania seeds in plastic tubes containing top soil and peat (2:1, v:v) spiked with various levels (0, 1000, 2000 mg Pb/kg dry soil) of lead nitrate. At six weeks after emergence, aqueous solutions of EDTA and/or HAc (in a 1:1 ratio with the metal) or distilled deionized water were applied to the root zones. Plants were harvested at 6 days after chelate addition to coincide with the duration of maximum metal solubility previously determined in this study. Results of the greenhouse experiment showed that coffeeweed was relatively tolerant to moderate levels of Pb and chelates as shown by very slight reductions in root and no discernable effects on shoot biomass. Root Pb concentrations increased with increasing levels of soil-applied Pb. Further increases in root Pb concentrations were attributed to chelate amendments. In the absence of chelates, translocation of Pb from roots to shoots was minimal. However, translocation dramatically increased in treatments with EDTA alone or in combination with HAc. Overall, the results of this study indicated that depending on the nature and type of Pb-contaminated soil being remediated, the bioavailability and uptake of Pb by coffeeweed can be enhanced by amending the soil with chelates especially after the plants have reached maximum biomass.
Figures


Similar articles
-
Bioavailability and uptake of lead by coffeeweed (Sesbania exaltata Raf.).Int J Environ Res Public Health. 2008 Dec;5(5):436-40. doi: 10.3390/ijerph5050436. Int J Environ Res Public Health. 2008. PMID: 19151440 Free PMC article.
-
Lead accumulation by tall fescue (Festuca arundinacea Schreb.) grown on a lead-contaminated soil.Int J Environ Res Public Health. 2005 Aug;2(2):228-33. doi: 10.3390/ijerph2005020005. Int J Environ Res Public Health. 2005. PMID: 16705822 Free PMC article.
-
Chelate-enhanced phytoextraction of lead-contaminated soils using coffeeweed (Sesbania exaltata Raf.).Bull Environ Contam Toxicol. 2002 Nov;69(5):624-31. doi: 10.1007/s00128-002-0106-6. Bull Environ Contam Toxicol. 2002. PMID: 12375108 No abstract available.
-
EDTA-assisted Pb phytoextraction.Chemosphere. 2009 Mar;74(10):1279-91. doi: 10.1016/j.chemosphere.2008.11.007. Epub 2009 Jan 1. Chemosphere. 2009. PMID: 19121533 Review.
-
Lead tolerance in plants: strategies for phytoremediation.Environ Sci Pollut Res Int. 2013 Apr;20(4):2150-61. doi: 10.1007/s11356-013-1485-4. Epub 2013 Jan 22. Environ Sci Pollut Res Int. 2013. PMID: 23338995 Review.
Cited by
-
Assisting Phytoremediation of Heavy Metals Using Chemical Amendments.Plants (Basel). 2019 Aug 21;8(9):295. doi: 10.3390/plants8090295. Plants (Basel). 2019. PMID: 31438507 Free PMC article. Review.
-
Bioavailability and uptake of lead by coffeeweed (Sesbania exaltata Raf.).Int J Environ Res Public Health. 2008 Dec;5(5):436-40. doi: 10.3390/ijerph5050436. Int J Environ Res Public Health. 2008. PMID: 19151440 Free PMC article.
References
-
- Kumar PBAN, Dushenkov V, Motto H, Raskin I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995;29(5):1232–1238. - PubMed
-
- Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997;31(3):860–865.
-
- Friedland AJ. In: Heavy metal tolerance in plants: Evolutionary aspects. Shaw AJ, editor. CRC Press; Boca Raton, FL: 1990.
-
- Glass DJ. Economic potential of phytoremediation. In: Raskin I, Ensley DD, editors. Phytoremediation of toxic metals: Using plants to clean up the environment. John Wiley & Sons, Inc; New York: 2000. pp. 1–14.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
