Conformational flexibility of metazoan fatty acid synthase enables catalysis
- PMID: 19151726
- PMCID: PMC2653270
- DOI: 10.1038/nsmb.1532
Conformational flexibility of metazoan fatty acid synthase enables catalysis
Abstract
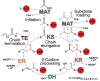
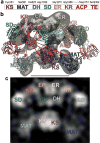
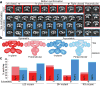
The metazoan cytosolic fatty acid synthase (FAS) contains all of the enzymes required for de novo fatty acid biosynthesis covalently linked around two reaction chambers. Although the three-dimensional architecture of FAS has been mostly defined, it is unclear how reaction intermediates can transfer between distant catalytic domains. Using single-particle EM, we have identified a near continuum of conformations consistent with a remarkable flexibility of FAS. The distribution of conformations was influenced by the presence of substrates and altered by different catalytic mutations, suggesting a direct correlation between conformation and specific enzymatic activities. We interpreted three-dimensional reconstructions by docking high-resolution structures of individual domains, and they show that the substrate-loading and condensation domains dramatically swing and swivel to access substrates within either reaction chamber. Concomitant rearrangement of the beta-carbon-processing domains synchronizes acyl chain reduction in one chamber with acyl chain elongation in the other.
Figures


References
-
- Sul HS, Smith S. Fatty acid synthesis in eukaryotes. In: Vance DE, V JE, editors. Biochemistry of lipids, lipoproteins and membranes. Elsevier; 2008. pp. 155–190.
-
- Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–81. - PubMed
-
- Jenni S, et al. Structure of fungal fatty acid synthase and implications for iterative substrate shuttling. Science. 2007;316:254–61. - PubMed
-
- Lomakin IB, Xiong Y, Steitz TA. The crystal structure of yeast fatty acid synthase, a cellular machine with eight active sites working together. Cell. 2007;129:319–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

