Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1alpha levels contribute to emergence of an aggressive phenotype in prostate cancer
- PMID: 19151763
- PMCID: PMC2651999
- DOI: 10.1038/onc.2008.476
Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1alpha levels contribute to emergence of an aggressive phenotype in prostate cancer
Retraction in
-
Retraction Note: Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1α levels contribute to emergence of an aggressive phenotype in prostate cancer.Oncogene. 2022 Jun;41(24):3383. doi: 10.1038/s41388-022-02357-z. Oncogene. 2022. PMID: 35589952 Free PMC article. No abstract available.
Abstract
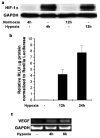
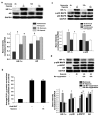
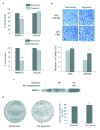
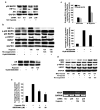
Androgen receptor (AR) signaling is involved in the development and progression of prostate cancer. Tumor microvasculature contributes to continual exposure of prostate cancer cells to hypoxia-reoxygenation, however, the role of hypoxia-reoxygenation in prostate cancer progression and modulation of AR signaling is not understood. In this study, we evaluated the effects of hypoxia-reoxygenation in LNCaP cells, a line of hormone responsive human prostate cancer cells. Our results demonstrate that hypoxia-reoxygenation resulted in increased survival, higher clonogenicity and enhanced invasiveness of these cells. Moreover, hypoxia-reoxygenation was associated with an increased AR activity independent of androgens as well as increased hypoxia inducible factor (HIF-1alpha) levels and activity. We also observed that the activation of p38 mitogen-activated protein (MAP) kinase pathway was an early response to hypoxia, and inhibition of p38 MAP kinase pathway by variety of approaches abolished hypoxia-reoxygenation induced increased AR activity as well as increased survival, clonogenicity and invasiveness. These results demonstrate a critical role for hypoxia-induced p38 MAP kinase pathway in androgen-independent AR activation in prostate cancer cells, and suggest that hypoxia-reoxygenation may select for aggressive androgen-independent prostate cancer phenotype.
Figures
Similar articles
-
Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1α in a low androgen environment.J Steroid Biochem Mol Biol. 2011 Jan;123(1-2):58-64. doi: 10.1016/j.jsbmb.2010.10.009. Epub 2010 Nov 5. J Steroid Biochem Mol Biol. 2011. PMID: 21056661
-
Coordinated action of hypoxia-inducible factor-1α and β-catenin in androgen receptor signaling.J Biol Chem. 2012 Sep 28;287(40):33594-606. doi: 10.1074/jbc.M112.388298. Epub 2012 Aug 3. J Biol Chem. 2012. PMID: 22865883 Free PMC article.
-
Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3'-kinase/protein kinase B in prostate cancer cells.Clin Cancer Res. 2003 Jul;9(7):2416-25. Clin Cancer Res. 2003. PMID: 12855613
-
Hypoxia-inducible factor-1 in human breast and prostate cancer.Endocr Relat Cancer. 2006 Sep;13(3):739-49. doi: 10.1677/erc.1.00728. Endocr Relat Cancer. 2006. PMID: 16954428 Review.
-
Androgen receptor involvement in the progression of prostate cancer.Endocr Relat Cancer. 2003 Jun;10(2):209-16. doi: 10.1677/erc.0.0100209. Endocr Relat Cancer. 2003. PMID: 12790784 Review.
Cited by
-
Kinomic profile in patient-derived glioma cells during hypoxia reveals c-MET-PI3K dependency for adaptation.Theranostics. 2021 Mar 5;11(11):5127-5142. doi: 10.7150/thno.54741. eCollection 2021. Theranostics. 2021. PMID: 33859738 Free PMC article.
-
Evaluation of the therapeutic potential of the selective p38 MAPK inhibitor Skepinone-L and the dual p38/JNK 3 inhibitor LN 950 in experimental K/BxN serum transfer arthritis.Inflammopharmacology. 2019 Dec;27(6):1217-1227. doi: 10.1007/s10787-019-00593-6. Epub 2019 Apr 29. Inflammopharmacology. 2019. PMID: 31037574
-
Marine alkaloid monanchoxymycalin C: a new specific activator of JNK1/2 kinase with anticancer properties.Sci Rep. 2020 Aug 6;10(1):13178. doi: 10.1038/s41598-020-69751-z. Sci Rep. 2020. PMID: 32764580 Free PMC article.
-
The nrf1 and nrf2 balance in oxidative stress regulation and androgen signaling in prostate cancer cells.Cancers (Basel). 2010 Jun 21;2(2):1354-78. doi: 10.3390/cancers2021354. Cancers (Basel). 2010. PMID: 24281119 Free PMC article.
-
Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties.Oncogene. 2016 Nov 17;35(46):5963-5976. doi: 10.1038/onc.2015.498. Epub 2016 Jan 25. Oncogene. 2016. PMID: 26804168 Free PMC article.
References
-
- Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, et al. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548–6554. - PubMed
-
- Asirvatham AJ, Schmidt M, Gao B, Chaudhary J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology. 2006;147:257–271. - PubMed
-
- Baek SH, Lee UY, Park EM, Han MY, Lee YS, Park YM. Role of protein kinase C delta in transmitting hypoxia signal to HSF and HIF-1. J Cell Physiol. 2001;188:223–235. - PubMed
-
- Berra E, Pagès G, Pouysségur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000;19:139–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

