Expression of HMP/AN2, a melanoma associated antigen, in murine cerebral gliomas: potential for radioimmunotargeting
- PMID: 19152070
- PMCID: PMC2741310
- DOI: 10.1007/s11060-009-9798-3
Expression of HMP/AN2, a melanoma associated antigen, in murine cerebral gliomas: potential for radioimmunotargeting
Abstract
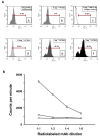
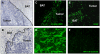
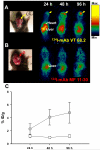
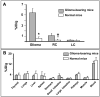
Human melanoma proteoglycan (HMP), a melanoma-associated antigen, is expressed in both human melanomas and gliomas. We used HMP-specific monoclonal antibody (mAb) VT68.2 to investigate whether murine GL261 cerebral gliomas express the HMP homologue AN2 and to determine whether AN2 could be targeted for selective delivery of radiation in vivo. HMP-specific mAb VT68.2 stained murine GL261 glioma cells grown in culture and intracerebrally in syngeneic C57BL/6 mice. Positron emission tomography with radiolabeled mAb VT68.2 showed high-contrast, positive images of gliomas against a negative background. At 96 h after injection, glioma uptake of radiolabeled mAb VT68.2 was 10x greater than that of the isotype control mAb and 20x greater than that detected in normal cerebral tissue. Our results show murine GL261 cerebral gliomas express AN2 and HMP-specific mAb VT68.2 accumulates selectively and specifically at high concentration and is retained within murine cerebral gliomas. Thus, HMP is a potential target for antibody-mediated selective delivery of radiation to cerebral gliomas in vivo.
Figures
Similar articles
-
Therapeutic efficacy of antiglioma mesenchymal extracellular matrix 131I-radiolabeled murine monoclonal antibody in a human glioma xenograft model.Cancer Res. 1988 Feb 1;48(3):559-66. Cancer Res. 1988. PMID: 2446747
-
Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model.Cancer Res. 2003 Dec 1;63(23):8487-91. Cancer Res. 2003. PMID: 14679014
-
Dosimetry and radiographic analysis of 131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: a phase II study.J Nucl Med. 2005 Jun;46(6):1042-51. J Nucl Med. 2005. PMID: 15937318 Clinical Trial.
-
AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration.J Neurocytol. 2002 Jul-Aug;31(6-7):497-505. doi: 10.1023/a:1025743731306. J Neurocytol. 2002. PMID: 14501219 Review.
-
The value of metabolic imaging in diagnosis and resection of cerebral gliomas.Nat Clin Pract Neurol. 2005 Dec;1(2):62-3. doi: 10.1038/ncpneuro0043. Nat Clin Pract Neurol. 2005. PMID: 16932494 Review. No abstract available.
Cited by
-
Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model.Cancer Immunol Immunother. 2011 Feb;60(2):153-60. doi: 10.1007/s00262-010-0946-6. Epub 2010 Dec 1. Cancer Immunol Immunother. 2011. PMID: 21120655 Free PMC article. Review.
-
Genetic Alterations in Gliomas Remodel the Tumor Immune Microenvironment and Impact Immune-Mediated Therapies.Front Oncol. 2021 Jun 8;11:631037. doi: 10.3389/fonc.2021.631037. eCollection 2021. Front Oncol. 2021. PMID: 34168976 Free PMC article. Review.
-
Immunocompetent murine models for the study of glioblastoma immunotherapy.J Transl Med. 2014 Apr 29;12:107. doi: 10.1186/1479-5876-12-107. J Transl Med. 2014. PMID: 24779345 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. - PubMed
-
- Fenstermaker RA, Ciesielski MJ. Immunotherapeutic strategies for malignant glioma. Cancer Control. 2004;11:181–191. - PubMed
-
- Kurpad SN, Zhao XG, Wikstrand CJ, Batra SK, McLendon RE, Bigner DD. Tumor antigens in astrocytic gliomas. Glia. 1995;15:244–256. - PubMed
-
- Sasaki M, Nakahira K, Kawano Y, et al. MAGE-E1, a new member of the melanoma-associated antigen gene family and its expression in human glioma. Cancer Res. 2001;61:4809–4814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

