Generation of single-chain LAGLIDADG homing endonucleases from native homodimeric precursor proteins
- PMID: 19153140
- PMCID: PMC2655683
- DOI: 10.1093/nar/gkp004
Generation of single-chain LAGLIDADG homing endonucleases from native homodimeric precursor proteins
Abstract
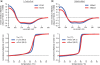
Homing endonucleases (HEs) cut long DNA target sites with high specificity to initiate and target the lateral transfer of mobile introns or inteins. This high site specificity of HEs makes them attractive reagents for gene targeting to promote DNA modification or repair. We have generated several hundred catalytically active, monomerized versions of the well-characterized homodimeric I-CreI and I-MsoI LAGLIDADG family homing endonuclease (LHE) proteins. Representative monomerized I-CreI and I-MsoI proteins (collectively termed mCreIs or mMsoIs) were characterized in detail by using a combination of biochemical, biophysical and structural approaches. We also demonstrated that both mCreI and mMsoI proteins can promote cleavage-dependent recombination in human cells. The use of single chain LHEs should simplify gene modification and targeting by requiring the expression of a single small protein in cells, rather than the coordinate expression of two separate protein coding genes as is required when using engineered heterodimeric zinc finger or homing endonuclease proteins.
Figures





References
-
- Stoddard BL. Homing endonuclease structure and function. Quart. Rev. Biophys. 2005;38:49–95. - PubMed
-
- Belfort M, Perlman PS. Mechanisms of intron mobility. J. Biol. Chem. 1995;270:30237–30240. - PubMed
-
- Argast GM, Stephens KM, Emond MJ, Monnat RJ., Jr I-PpoI and I-CreI homing site sequence degeneracy determined by random mutagenesis and sequential in vitro enrichment. J. Mol. Biol. 1998;280:345–353. - PubMed
-
- Chevalier B, Turmel M, Lemieux C, Monnat RJ, Jr, Stoddard BL. Flexible DNA target site recognition by divergent homing endonuclease isoschizomers I-CreI and I-MsoI. J. Mol. Biol. 2003;329:253–269. - PubMed
-
- Silva GH, Dalgaard JZ, Belfort M, Van Roey P. Crystal structure of the thermostable archaeal intron-encoded endonuclease I-DmoI. J. Mol. Biol. 1999;286:1123–1136. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

