Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection
- PMID: 19153236
- PMCID: PMC2655566
- DOI: 10.1128/JVI.01796-08
Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection
Abstract
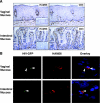
Mucosal surfaces play a major role in human immunodeficiency virus type 1 (HIV-1) transmission and pathogenesis, and yet the role of lamina propria macrophages in mucosal HIV-1 infection has received little investigative attention. We report here that vaginal and intestinal macrophages display distinct phenotype and HIV-1 permissiveness profiles. Vaginal macrophages expressed the innate response receptors CD14, CD89, CD16, CD32, and CD64 and the HIV-1 receptor/coreceptors CD4, CCR5, and CXCR4, similar to monocytes. Consistent with this phenotype, green fluorescent protein-tagged R5 HIV-1 entered macrophages in explanted vaginal mucosa as early as 30 min after inoculation of virus onto the epithelium, and purified vaginal macrophages supported substantial levels of HIV-1 replication by a panel of highly macrophage-tropic R5 viruses. In sharp contrast, intestinal macrophages expressed no detectable, or very low levels of, innate response receptors and HIV-1 receptor/coreceptors and did not support HIV-1 replication, although virus occasionally entered macrophages in intestinal tissue explants. Thus, vaginal, but not intestinal, macrophages are monocyte-like and permissive to R5 HIV-1 after the virus has translocated across the epithelium. These findings suggest that genital and gut macrophages have different roles in mucosal HIV-1 pathogenesis and that vaginal macrophages play a previously underappreciated but potentially important role in mucosal HIV-1 infection in the female genital tract.
Figures




References
-
- Alexander, N. J. 1990. Sexual transmission of human immunodeficiency virus: virus entry into the male and female genital tract. Fertil. Steril. 541-18. - PubMed
-
- Boyaka, P. N., J. R. McGhee, C. Czerkinsky, and J. Mestecky. 2005. Mucosal vaccines: an overview, p. 855-873. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, J. R. McGhee, and W. Strober (ed.), Mucosal Immunology, 3rd ed. Elsevier/Academic Press, Amsterdam, The Netherlands.
-
- Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. - PMC - PubMed
-
- Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6475-479. - PubMed
-
- Crowe, S., T. Zhu, and W. A. Muller. 2003. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J. Leukoc. Biol. 74635-641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-61297/DK/NIDDK NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- T32 AI007051/AI/NIAID NIH HHS/United States
- DK-47322/DK/NIDDK NIH HHS/United States
- RR-20136/RR/NCRR NIH HHS/United States
- C06 RR020136/RR/NCRR NIH HHS/United States
- R21 DK074033/DK/NIDDK NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- R01 DK047322/DK/NIDDK NIH HHS/United States
- DK-64400/DK/NIDDK NIH HHS/United States
- R01 MH064408/MH/NIMH NIH HHS/United States
- R01 DK061297/DK/NIDDK NIH HHS/United States
- DK-54495/DK/NIDDK NIH HHS/United States
- R24 DK064400/DK/NIDDK NIH HHS/United States
- DK-74033/DK/NIDDK NIH HHS/United States
- T32 AI-007051/AI/NIAID NIH HHS/United States
- K24 DK068389/DK/NIDDK NIH HHS/United States
- R01 DK054495/DK/NIDDK NIH HHS/United States
- AI-67854/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

