Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7
- PMID: 19153603
- PMCID: PMC2646152
- DOI: 10.1038/emboj.2008.294
Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7
Abstract
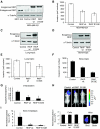
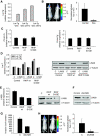
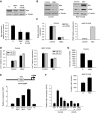
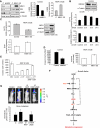
Raf kinase inhibitory protein (RKIP) negatively regulates the MAP kinase (MAPK), G protein-coupled receptor kinase-2, and NF-kappaB signalling cascades. RKIP has been implicated as a metastasis suppressor for prostate cancer, but the mechanism is not known. Here, we show that RKIP inhibits invasion by metastatic breast cancer cells and represses breast tumour cell intravasation and bone metastasis in an orthotopic murine model. The mechanism involves inhibition of MAPK, leading to decreased transcription of LIN28 by Myc. Suppression of LIN28 enables enhanced let-7 processing in breast cancer cells. Elevated let-7 expression inhibits HMGA2, a chromatin remodelling protein that activates pro-invasive and pro-metastatic genes, including Snail. LIN28 depletion and let-7 expression suppress bone metastasis, and LIN28 restores bone metastasis in mice bearing RKIP-expressing breast tumour cells. These results indicate that RKIP suppresses invasion and metastasis in part through a signalling cascade involving MAPK, Myc, LIN28, let-7, and downstream let-7 targets. RKIP regulation of two pluripotent stem cell genes, Myc and LIN28, highlights the importance of RKIP as a key metastasis suppressor and potential therapeutic agent.
Figures

References
-
- Akaishi J, Onda M, Asaka S, Okamoto J, Miyamoto S, Nagahama M, Ito K, Kawanami O, Shimizu K (2006) Growth-suppressive function of phosphatidylethanolamine-binding protein in anaplastic thyroid cancer. Anticancer Res 26: 4437–4442 - PubMed
-
- Al-Mulla F, Hagan S, Behbehani AI, Bitar MS, George SS, Going JJ, Garcia JJ, Scott L, Fyfe N, Murray GI, Kolch W (2006) Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. J Clin Oncol 24: 5672–5679 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

