Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4
- PMID: 19153612
- PMCID: PMC2637330
- DOI: 10.1038/emboj.2008.267
Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4
Abstract
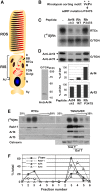
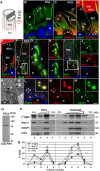
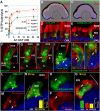
Dysfunctions of primary cilia and cilia-derived sensory organelles underlie a multitude of human disorders, including retinal degeneration, yet membrane targeting to the cilium remains poorly understood. Here, we show that the newly identified ciliary targeting VxPx motif present in rhodopsin binds the small GTPase Arf4 and regulates its association with the trans-Golgi network (TGN), which is the site of assembly and function of a ciliary targeting complex. This complex is comprised of two small GTPases, Arf4 and Rab11, the Rab11/Arf effector FIP3, and the Arf GTPase-activating protein ASAP1. ASAP1 mediates GTP hydrolysis on Arf4 and functions as an Arf4 effector that regulates budding of post-TGN carriers, along with FIP3 and Rab11. The Arf4 mutant I46D, impaired in ASAP1-mediated GTP hydrolysis, causes aberrant rhodopsin trafficking and cytoskeletal and morphological defects resulting in retinal degeneration in transgenic animals. As the VxPx motif is present in other ciliary membrane proteins, the Arf4-based targeting complex is most likely a part of conserved machinery involved in the selection and packaging of the cargo destined for delivery to the cilium.
Figures


Similar articles
-
Molecular assemblies that control rhodopsin transport to the cilia.Vision Res. 2012 Dec 15;75:5-10. doi: 10.1016/j.visres.2012.07.015. Epub 2012 Aug 7. Vision Res. 2012. PMID: 22892112 Free PMC article. Review.
-
The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting.J Cell Sci. 2015 Apr 1;128(7):1375-85. doi: 10.1242/jcs.162925. Epub 2015 Feb 11. J Cell Sci. 2015. PMID: 25673879 Free PMC article.
-
The Arf GEF GBF1 and Arf4 synergize with the sensory receptor cargo, rhodopsin, to regulate ciliary membrane trafficking.J Cell Sci. 2017 Dec 1;130(23):3975-3987. doi: 10.1242/jcs.205492. Epub 2017 Oct 12. J Cell Sci. 2017. PMID: 29025970 Free PMC article.
-
The ins and outs of the Arf4-based ciliary membrane-targeting complex.Small GTPases. 2021 Jan;12(1):1-12. doi: 10.1080/21541248.2019.1616355. Epub 2019 May 17. Small GTPases. 2021. PMID: 31068062 Free PMC article. Review.
-
A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1.Mol Biol Cell. 2011 Sep;22(18):3289-305. doi: 10.1091/mbc.E11-01-0082. Epub 2011 Jul 20. Mol Biol Cell. 2011. PMID: 21775626 Free PMC article.
Cited by
-
Clustering of Rab11 vesicles in influenza A virus infected cells creates hotspots containing the 8 viral ribonucleoproteins.Small GTPases. 2017 Apr 3;8(2):71-77. doi: 10.1080/21541248.2016.1199190. Epub 2016 Jun 23. Small GTPases. 2017. PMID: 27337591 Free PMC article. Review.
-
Molecular assemblies that control rhodopsin transport to the cilia.Vision Res. 2012 Dec 15;75:5-10. doi: 10.1016/j.visres.2012.07.015. Epub 2012 Aug 7. Vision Res. 2012. PMID: 22892112 Free PMC article. Review.
-
Crosstalk of small GTPases at the Golgi apparatus.Small GTPases. 2012 Apr-Jun;3(2):80-90. doi: 10.4161/sgtp.19842. Small GTPases. 2012. PMID: 22790194 Free PMC article. Review.
-
Defects in the Exocyst-Cilia Machinery Cause Bicuspid Aortic Valve Disease and Aortic Stenosis.Circulation. 2019 Oct 15;140(16):1331-1341. doi: 10.1161/CIRCULATIONAHA.119.038376. Epub 2019 Aug 7. Circulation. 2019. PMID: 31387361 Free PMC article.
-
Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments.J Cell Sci. 2009 Jun 15;122(Pt 12):2003-13. doi: 10.1242/jcs.039982. Epub 2009 May 19. J Cell Sci. 2009. PMID: 19454479 Free PMC article.
References
-
- Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA (2002) Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci 43: 3027–3036 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

