Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases
- PMID: 19154146
- PMCID: PMC2765506
- DOI: 10.1021/ar800201v
Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases
Abstract
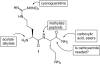
Nitric oxide (NO), which is produced from L-arginine by the nitric oxide synthase (NOS) family of enzymes, is an important second-messenger molecule that regulates several physiological functions. In endothelial cells, it relaxes smooth muscle, which decreases blood pressure. Macrophage cells produce NO as an immune defense system to destroy pathogens and microorganisms. In neuronal cells, NO controls the release of neurotransmitters and is involved in synaptogenesis, synaptic plasticity, memory function, and neuroendocrine secretion. NO is a free radical that is commonly thought to contribute to oxidative damage and molecule and tissue destruction, and thus it is somewhat surprising that it has so many significant beneficial physiological effects. However, the cell is generally protected from NO's toxic effects, except under certain pathological conditions in which excessive NO is produced. In that case, tissue damage and oxidative stress can result, leading to a wide variety of diseases, including rheumatoid arthritis, Alzheimer's disease, and Parkinson's disease, among others. In this Account, we describe research aimed at identifying small molecules that can selectively inhibit only the neuronal isozyme of NOS, nNOS. By targeting only nNOS, we attained the beneficial effects of lowering excess NO in the brain without the detrimental effects of inhibition of the two isozymes found elsewhere in the body (eNOS and iNOS). Initially, in pursuit of this goal, we sought to identify differences in the second sphere of amino acids in the active site of the isozymes. From this study, the first class of dual nNOS-selective inhibitors was identified. The moieties important for selectivity in the best lead compound were determined by structure modification. Enhancement provided highly potent, nNOS-selective dipeptide amides and peptidomimetics, which were active in a rabbit model for fetal neurodegeneration. Crystal structures of these compounds bound to NOS isozymes showed a one-amino-acid difference between nNOS and eNOS in the second sphere of amino acids; this was the difference that we were searching for from the beginning of this project. With the aid of these crystal structures, we developed a new fragment-based de novo design method called "fragment hopping", which allowed the design of a new class of nonpeptide nNOS-selective inhibitors. These compounds were modified to give low nanomolar, highly dual-selective nNOS inhibitors, which we recently showed are active in a rabbit model for the prevention of neurobehavioral symptoms of cerebral palsy. These compounds could also have general application in other neurodegenerative diseases for which excess NO is responsible.
Figures

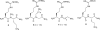
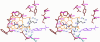
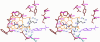



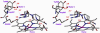
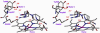
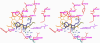
References
-
- Li H, Poulos TL. Structure-function studies on nitric oxide synthases. J. Inorg. Biochem. 2005;99(1):293–305. - PubMed
- Fleming I. Biology of nitric oxide synthases. Handbook of Physiology: Microcirculation. (2nd Edition) 2008:56–80.
-
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239. - PubMed
- Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408. - PubMed
- Ferriero DM, Holtzman DM, Black SM, Sheldon RA. Neonatal mice lacking neuronal nitric oxide synthase are less vulnerable to hypoxic-ischemic injury. Neurobiol. Dis. 1996;3:64–71. - PubMed
-
- Erdal EP, Litzinger EA, Seo J, Zhu Y, Ji H, Silverman RB. Selective neuronal nitric oxide synthase inhibitors. Curr. Topics Med. Chem. 2005;5:603–624. - PubMed
-
- Furfine ES, Harmon MF, Paith JE, Garvey EP. Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry. 1993;32:8512–8517. - PubMed
-
- Yamamoto K, Shimamura K, Sekiguchi F, Sunano S. Effects of NG-nitro-L-arginine on the blood pressure of spontaneously hypertensive rats with different degrees of hypertension. Clin. Exp. Hypertens. 2001;23(7):533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

